Abstract
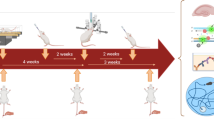
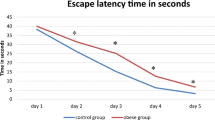
Prenatal stress, including maternal immune activation (MIA), affects cognitive performance in the offspring. Since insulin could improve cognitive function in several aspects, we hypothesized that intranasal insulin would attenuate MIA-induced learning and memory deficits. In the present study, the pregnant Wistar rats received lipopolysaccharide (LPS, 250 µg/kg) intraperitoneally on gestational day 15. Intranasal insulin (2 IU, 7 days) was administered to male pups from PND 34–47. During late adolescence, the Morris Water Maze and in vivo electrophysiological recording were performed in male rats to assess spatial learning and memory and long-term potentiation (LTP), respectively. Also, the hippocampal expression of BDNF and PSD-95 was evaluated using real-time PCR. Our results demonstrated that MIA impaired spatial learning and memory in the male pups. Hippocampal synaptic plasticity was also impaired in the adolescent male rats. However, intranasal administration of insulin could overcome MIA-induced impairments and improve learning, memory, and synaptic plasticity in the male pups. Although BDNF and PSD-95 levels were not altered in the hippocampus of MIA pups, intranasal insulin increased PSD-95 expression. Taken together, these findings suggest that intranasal insulin promotes cognitive performance in MIA-exposed pups during adolescence; however, the underlying molecular mechanisms remain to be elucidated.
Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
O’Connor, T. G. & Ciesla, A. A. Maternal immune activation hypotheses for human neurodevelopment: some outstanding questions. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 7 (5), 471–479 (2022).
Csatlosova, K. et al. Maternal immune activation in rats attenuates the excitability of monoamine-secreting neurons in adult offspring in a sex-specific way. Eur. Neuropsychopharmacol. 43, 82–91 (2021).
Al-Haddad, B. J. S. et al. Long-term risk of neuropsychiatric disease after exposure to infection in utero. JAMA Psychiatry. 76 (6), 594–602 (2019).
Atladóttir, H. O. et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 40 (12), 1423–1430 (2010).
Lee, B. K. et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav. Immun. 44, 100–105 (2015).
Lydholm, C. N. et al. Parental infections Before, During, and after pregnancy as risk factors for mental disorders in childhood and adolescence: A nationwide Danish study. Biol. Psychiatry. 85 (4), 317–325 (2019).
Vlasova, R. M. et al. Maternal immune activation during pregnancy alters postnatal brain growth and cognitive development in nonhuman primate offspring. J. Neurosci. 41 (48), 9971–9987 (2021).
Couch, A. C. M. et al. Maternal immune activation primes deficiencies in adult hippocampal neurogenesis. Brain Behav. Immun. 97, 410–422 (2021).
Knuesel, I. et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 10 (11), 643–660 (2014).
Smolders, S., Notter, T., Smolders, S. M. T., Rigo, J-M. & Brône, B. Controversies and prospects about microglia in maternal immune activation models for neurodevelopmental disorders. Brain Behav. Immun. 73, 51–65 (2018).
Matteoli, M., Pozzi, D., Fossati, M. & Menna, E. Immune synaptopathies: how maternal immune activation impacts synaptic function during development. EMBO J. 42 (13), e113796 (2023).
Murray, K. N. et al. Evolution of a maternal immune activation (mIA) model in rats: early developmental effects. Brain Behav. Immun. 75, 48–59 (2019).
Oskvig, D. B., Elkahloun, A. G., Johnson, K. R., Phillips, T. M. & Herkenham, M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav. Immun. 26 (4), 623–634 (2012).
Quagliato, L. A., de Matos, U. & Nardi, A. E. Maternal immune activation generates anxiety in offspring: A translational meta-analysis. Transl Psychiatry. 11 (1), 1–6 (2021).
Dutra, M. L. et al. Maternal immune activation induces autism-like behavior and reduces brain-derived neurotrophic factor levels in the hippocampus and offspring cortex of C57BL/6 mice. Neurosci Lett [Internet]. ;793:136974. (2023). Available from: https://www.sciencedirect.com/science/article/pii/S0304394022005353
Perez-Palomar, B., Erdozain, A. M., Erkizia-Santamaría, I., Ortega, J. E. & Meana, J. J. Maternal Immune Activation Induces Cortical Catecholaminergic Hypofunction and Cognitive Impairments in Offspring. J Neuroimmune Pharmacol [Internet]. ;18(3):348–65. (2023). Available from: https://doi.org/10.1007/s11481-023-10070-1
Zhang, Y-M. et al. Resveratrol ameliorates maternal immune activation-associated cognitive impairment in adult male offspring by relieving inflammation and improving synaptic dysfunction. Front. Behav. Neurosci. 17, 1271653 (2023).
Glass, R., Norton, S., Fox, N. & Kusnecov, A. W. Maternal immune activation with Staphylococcal enterotoxin A produces unique behavioral changes in C57BL/6 mouse offspring. Brain Behav. Immun. 75, 12–25 (2019).
Khan, D. et al. Long-term effects of maternal immune activation on depression-like behavior in the mouse. Transl Psychiatry. 4 (2), e363 (2014).
Sal-Sarria, S., Conejo, N. M. & González-Pardo, H. Maternal immune activation and its multifaceted effects on learning and memory in rodent offspring: A systematic review. Neurosci. Biobehav Rev. 164, 105844 (2024).
Zhao, Q. et al. Maternal immune activation-induced PPARγ-dependent dysfunction of microglia associated with neurogenic impairment and aberrant postnatal behaviors in offspring. Neurobiol. Dis. 125, 1–13 (2019).
Schaafsma, W. et al. Maternal inflammation induces immune activation of fetal microglia and leads to disrupted microglia immune responses, behavior, and learning performance in adulthood. Neurobiol. Dis. 106, 291–300 (2017).
Talukdar, P. M. et al. Maternal immune activation causes Schizophrenia-like behaviors in the offspring through activation of immune-Inflammatory, oxidative and apoptotic Pathways, and Lowered antioxidant defenses and neuroprotection. Mol. Neurobiol. 57 (10), 4345–4361 (2020).
Giovanoli, S., Weber-Stadlbauer, U., Schedlowski, M., Meyer, U. & Engler, H. Prenatal immune activation causes hippocampal synaptic deficits in the absence of overt microglia anomalies. Brain Behav. Immun. 55, 25–38 (2016).
Andoh, M. et al. Exercise reverses behavioral and synaptic abnormalities after maternal inflammation. Cell. Rep. 27 (10), 2817–2825e5 (2019).
Yan S, Wang L, Samsom JN, Ujic D, Liu F. PolyI:C Maternal Immune Activation on E9.5 Causes the Deregulation of Microglia and the Complement System in Mice, Leading to Decreased Synaptic Spine Density. Int J Mol Sci. 2024 May 17;25(10):5480. doi: 10.3390/ijms25105480. PMID: 38791517; PMCID: PMC11121703.
Bourgeron, T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat. Rev. Neurosci. 16 (9), 551–563 (2015).
Sheibani, V. et al. The effects of neurosteroid allopregnanolone on synaptic dysfunction in the hippocampus in experimental parkinsonism rats: an electrophysiological and molecular study. Neuropeptides 92, 102229 (2022).
Joushi, S., Esmaeilpour, K., Masoumi-Ardakani, Y., Esmaeili-Mahani, S. & Sheibani, V. Effects of short environmental enrichment on early-life adversity induced cognitive alternations in adolescent rats. J. Neurosci. Res. 99 (12), 3373–3391 (2021).
Morris, R. G. M. Long-term potentiation and memory. Philos. Trans. R Soc. Lond. Ser. B Biol. Sci. 358 (1432), 643–647 (2003).
Kowiański, P. et al. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 38 (3), 579–593 (2018).
Colucci-D'Amato L, Speranza L, Volpicelli F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int J Mol Sci. 2020 Oct 21;21(20):7777. doi: 10.3390/ijms21207777. PMID: 33096634; PMCID: PMC7589016.
Levy AM, Gomez-Puertas P, Tümer Z. Neurodevelopmental Disorders Associated with PSD-95 and Its Interaction Partners. Int J Mol Sci. 2022 Apr 15;23(8):4390. doi: 10.3390/ijms23084390. PMID: 35457207; PMCID: PMC9025546.
Coley, A. A. & Gao, W-J. PSD95: A synaptic protein implicated in schizophrenia or autism? Prog Neuropsychopharmacol. Biol. Psychiatry. 82, 187–194 (2018).
Woods, R. M. et al. Maternal immune activation and role of placenta in the prenatal programming of neurodevelopmental disorders. Neuronal Signal. 7 (2), NS20220064 (2023).
Craft, S. et al. Safety, Efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and alzheimer disease dementia: A randomized clinical trial. JAMA Neurol. 77 (9), 1099–1109 (2020).
Lv, H. et al. Intranasal insulin administration May be highly effective in improving cognitive function in mice with cognitive dysfunction by reversing brain insulin resistance. Cogn. Neurodyn. 14 (3), 323–338 (2020).
Rajasekar, N., Nath, C., Hanif, K. & Shukla, R. Intranasal insulin administration ameliorates streptozotocin (ICV)-Induced insulin receptor Dysfunction, Neuroinflammation, Amyloidogenesis, and memory impairment in rats. Mol. Neurobiol. 54 (8), 6507–6522 (2017).
Reger, M. A. et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70 (6), 440–448 (2008).
Benedict, C. et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29 (10), 1326–1334 (2004).
Benedict, C., Kern, W., Schultes, B., Born, J. & Hallschmid, M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J. Clin. Endocrinol. Metab. 93 (4), 1339–1344 (2008).
Benedict, C. et al. Intranasal insulin improves memory in humans: superiority of insulin Aspart. Neuropsychopharmacol. Off Publ Am. Coll. Neuropsychopharmacol. 32 (1), 239–243 (2007).
Reger, M. A. et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J. Alzheimers Dis. 13 (3), 323–331 (2008).
Yang, L. et al. Intranasal insulin ameliorates cognitive impairment in a rat model of parkinson’s disease through Akt/GSK3β signaling pathway. Life Sci. 259, 118159 (2020).
Mao, Y-F. et al. Intranasal insulin alleviates cognitive deficits and amyloid pathology in young adult APPswe/PS1dE9 mice. Aging Cell. 15 (5), 893–902 (2016).
Ramos-Rodriguez, J. J. et al. Intranasal insulin reverts central pathology and cognitive impairment in diabetic mother offspring. Mol. Neurodegener. 12 (1), 57 (2017).
Smith, C. J. et al. Intranasal insulin helps overcome brain insulin deficiency and improves survival and post-stroke cognitive impairment in male mice. J. Neurosci. Res. 101 (11), 1757–1769 (2023).
Núñez Estevez, K. J., Rondón-Ortiz, A. N., Nguyen, J. Q. T. & Kentner, A. C. Environmental influences on placental programming and offspring outcomes following maternal immune activation. Brain Behav Immun [Internet]. ;83:44–55. (2020). Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85073165171&doi=10.1016%2Fj.bbi.2019.08.192&partnerID=40&md5=5c284b925870a29a2fd9f09208adda22
Kalish, B. T. et al. Maternal immune activation in mice disrupts proteostasis in the fetal brain. Nat. Neurosci. 24 (2), 204–213 (2021).
Wang, Y., Fu, A. K. Y. & Ip, N. Y. Instructive roles of astrocytes in hippocampal synaptic plasticity: neuronal activity-dependent regulatory mechanisms. FEBS J. 289 (8), 2202–2218 (2022).
Tolias, K. F., Duman, J. G. & Um, K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog Neurobiol. 94 (2), 133–148 (2011).
Hao, L. Y., Hao, X. Q., Li, S. H. & Li, X. H. Prenatal exposure to lipopolysaccharide results in cognitive deficits in age-increasing offspring rats. Neuroscience [Internet]. ;166(3):763–70. (2010). Available from: https://www.sciencedirect.com/science/article/pii/S0306452210000072
Zhang, T., Dolga, A. M., Eisel, U. L. M. & Schmidt, M. Novel crosstalk mechanisms between GluA3 and Epac2 in synaptic plasticity and memory in alzheimer’s disease. Neurobiol. Dis. 191, 106389 (2024).
Cieślik M, Gąssowska-Dobrowolska M, Jęśko H, Czapski GA, Wilkaniec A, Zawadzka A, Dominiak A, Polowy R, Filipkowski RK, Boguszewski PM, Gewartowska M, Frontczak-Baniewicz M, Sun GY, Beversdorf DQ, Adamczyk A. Maternal Immune Activation Induces Neuroinflammation and Cortical Synaptic Deficits in the Adolescent Rat Offspring. Int J Mol Sci. 2020 Jun 8;21(11):4097. doi: 10.3390/ijms21114097. PMID: 32521803; PMCID: PMC7312084.
Vallejo, D., Codocedo, J. F. & Inestrosa, N. C. Posttranslational modifications regulate the postsynaptic localization of PSD-95. Mol. Neurobiol. 54 (3), 1759–1776 (2017).
Forrest, C. M. et al. Prenatal activation of Toll-like receptors-3 by administration of the viral mimetic poly(I:C) changes synaptic proteins, N-methyl-D-aspartate receptors and neurogenesis markers in offspring. Mol. Brain. 5, 22 (2012).
Schirmbeck, G. H. et al. Long-term LPS systemic administration leads to memory impairment and disturbance in astrocytic homeostasis. Neurotoxicology 99, 322–331 (2023).
Das, S., Mishra, K. P., Ganju, L. & Singh, S. B. Andrographolide - A promising therapeutic agent, negatively regulates glial cell derived neurodegeneration of prefrontal cortex, hippocampus and working memory impairment. J. Neuroimmunol. 313, 161–175 (2017).
Hemmerle, A. M. et al. Modulation of schizophrenia-related genes in the forebrain of adolescent and adult rats exposed to maternal immune activation. Schizophr Res. 168 (1–2), 411–420 (2015).
Han, M., Zhang, J-C., Huang, X-F. & Hashimoto, K. Intake of 7,8-dihydroxyflavone from pregnancy to weaning prevents cognitive deficits in adult offspring after maternal immune activation. Eur. Arch. Psychiatry Clin. Neurosci. 267 (5), 479–483 (2017).
Cieślik, M. et al. The synaptic dysregulation in adolescent rats exposed to maternal immune activation. Front. Mol. Neurosci. 13, 555290 (2020).
de la Monte, S. M. Intranasal insulin therapy for cognitive impairment and neurodegeneration: current state of the Art. Expert Opin. Drug Deliv. 10 (12), 1699–1709 (2013).
Zhao, F., Siu, J. J., Huang, W., Askwith, C. & Cao, L. Insulin modulates excitatory synaptic transmission and synaptic plasticity in the mouse hippocampus. Neuroscience 411, 237–254 (2019).
Barutçu, Ö. et al. Insulin-induced long-term potentiation in the dentate gyrus of hippocampal formation. Psychoneuroendocrinology 157, 106343 (2023).
van der Heide, L. P., Kamal, A., Artola, A., Gispen, W. H. & Ramakers, G. M. J. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J. Neurochem. 94 (4), 1158–1166 (2005).
Chen, Y. et al. Intranasal insulin prevents Anesthesia-Induced cognitive impairment and chronic neurobehavioral changes. Front. Aging Neurosci. 9, 136 (2017).
Maimaiti, S. et al. Intranasal insulin improves Age-Related cognitive deficits and reverses electrophysiological correlates of brain aging. J. Gerontol. Biol. Sci. Med. Sci. 71 (1), 30–39 (2016).
Roque, P. S. et al. Intranasal insulin rescues repeated anesthesia-induced deficits in synaptic plasticity and memory and prevents apoptosis in neonatal mice via mTORC1. Sci. Rep. 11 (1), 15490 (2021).
Yu, Q. et al. Intranasal insulin increases synaptic protein expression and prevents Anesthesia-Induced cognitive deficits through mTOR-eEF2 pathway. J. Alzheimers Dis. 70 (3), 925–936 (2019).
Rajasekar, N., Nath, C., Hanif, K. & Shukla, R. Intranasal insulin improves cerebral blood flow, Nrf-2 expression and BDNF in STZ (ICV)-induced memory impaired rats. Life Sci. 173, 1–10 (2017).
Simon, K. U. et al. da RP,. Intranasal insulin treatment modulates the neurotropic, inflammatory, and oxidant mechanisms in the cortex and hippocampus in a low-grade inflammation model. Peptides. ;123:170175. (2020).
Ivanov, A. D., Tukhbatova, G. R., Salozhin, S. V. & Markevich, V. A. NGF but not BDNF overexpression protects hippocampal LTP from beta-amyloid-induced impairment. Neuroscience 289, 114–122 (2015).
Cao, H. et al. High frequency repetitive transcranial magnetic stimulation alleviates cognitive deficits in 3xTg-AD mice by modulating the PI3K/Akt/GLT-1 axis. Redox Biol. 54, 102354 (2022).
Lee, C-C., Huang, C-C. & Hsu, K-S. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology 61 (4), 867–879 (2011).
Akhtar, A. & Sah, S. P. Insulin signaling pathway and related molecules: role in neurodegeneration and alzheimer’s disease. Neurochem Int. 135, 104707 (2020).
Joushi, S., Esmaeilpour, K., Masoumi-Ardakani, Y., Esmaeili-Mahani, S. & Sheibani, V. Intranasal Oxytocin administration facilitates the induction of long-term potentiation and promotes cognitive performance of maternally separated rats. Psychoneuroendocrinology 123, 105044 (2021).
Marks, D. R., Tucker, K., Cavallin, M. A., Mast, T. G. & Fadool, D. A. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J. Neurosci. Off J. Soc. Neurosci. 29 (20), 6734–6751 (2009).
Rehman, N. U. et al. Effect of 4-Fluoro-N-(4-sulfamoylbenzyl) benzene sulfonamide on cognitive deficits and hippocampal plasticity during nicotine withdrawal in rats. Biomed. Pharmacother. 131, 110783 (2020).
Joushi, S. et al. Maternal separation impairs mother’s cognition 1 month beyond the separation. Int. J. Dev. Neurosci. Off J. Int. Soc. Dev. Neurosci. 81 (7), 605–615 (2021).
Rajizadeh, M. A., Esmaeilpour, K., Haghparast, E., Ebrahimi, M. N. & Sheibani, V. Voluntary exercise modulates learning & memory and synaptic plasticity impairments in sleep deprived female rats. Brain Res. 1729, 146598 (2020).
Khodamoradi, M., Asadi-Shekaari, M., Esmaeili-Mahani, S., Esmaeilpour, K. & Sheibani, V. Effects of genistein on cognitive dysfunction and hippocampal synaptic plasticity impairment in an ovariectomized rat Kainic acid model of seizure. Eur. J. Pharmacol. 786, 1–9 (2016).
Shahraki, S. et al. Choline chloride modulates learning, memory, and synaptic plasticity impairments in maternally separated adolescent male rats. Int. J. Dev. Neurosci. Off J. Int. Soc. Dev. Neurosci. 82 (1), 19–38 (2022).
Salari, M. et al. Impact of sleep deprivation on the brain’s inflammatory response triggered by lipopolysaccharide and its consequences on Spatial learning and memory and Long-Term potentiation in male rats. Neuroimmunomodulation 31 (1), 12–24 (2024).
Fadaei-Kenarsary, M., Esmaeilpour, K., Shabani, M. & Sheibani, V. Chronic maternal morphine exposure and early-life adversity induce impairment in synaptic plasticity of adolescent male rats. Neurosci. Lett. 812, 137365 (2023).
Mohammadipoor-Ghasemabad, L., Sangtarash, M. H., Sheibani, V., Sasan, H. A. & Esmaeili-Mahani, S. Hippocampal microRNA-191a-5p regulates BDNF expression and shows correlation with cognitive impairment induced by Paradoxical sleep deprivation. Neuroscience 414, 49–59 (2019).
Acknowledgements
This study was supported by Neuroscience Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran.
Funding
In this project, funding for experiments was provided by Neuroscience Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran.
Author information
Authors and Affiliations
Author notes
Sara Joushi is the main corresponding author for this work.
- Sara Joushi
Contributions
Hadis.K. and Haniyeh.K. contributed to modeling, performed the behavioral experiments and molecular assessments, analyzed the data and wrote the manuscript. M.R. contributed to electrophysiological experiment. M.A. contributed to modeling and behavioral experiments. L.M. contributed to molecular assessments. S.J. designed and supervised the project and contributed to interpretation of the results, and revised the manuscript. V.Sh. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
All experiments were done in accordance with the ARRIVE guidelines and National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80 − 23, revised 1996). All experimental procedures were conducted in accordance with institutional and international guidelines for animal care, as approved by the Institutional Animal Research Ethics Committee of Kerman University of Medical Sciences (Ethics code: IR.KMU.AEC.1402.84).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kariminejad-Farsangi, H., Kariminejad-Farsangi, H., Rajizadeh, M.A. et al. Intranasal insulin ameliorates prenatal LPS-induced learning and memory impairments in adolescent male rats: A behavioral, electrophysiological, and molecular study. Sci Rep (2026). https://doi.org/10.1038/s41598-026-40163-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-40163-9