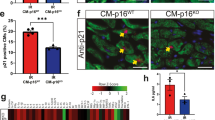
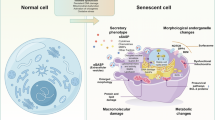
Abstract
Cardiovascular diseases remain the leading cause of global mortality. Cellular senescence has recently been implicated in the pathogenesis of various cardiovascular diseases. Our group has previously shown that the matricellular protein CCN5 is a potent anti-fibrotic molecule capable of inhibiting and reversing cardiac fibrosis. In this study, we investigated whether CCN5 can modulate cellular senescence in the heart utilizing three readouts: western blotting for p53 and p21, staining for senescence-associated β-galactosidase, and microscopic analysis of γH2AX-foci. CCN5 effectively inhibited doxorubicin-induced cellular senescence in both H9c2 cardiac myoblasts and fibroblasts. In addition, CCN5 suppressed cellular senescence in H9c2 cardiac myoblasts induced by the senescence-associated secretory phenotype factors secreted from cardiac fibroblast, and vice versa. CCN5 also restored the apoptotic response of senescent cells. Finally, CCN5 attenuated myocardial infarction-induced cellular senescence in mice. Collectively, our findings provide novel insights into the potential role of CCN5 in the development of anti-senescence therapies.
Similar content being viewed by others
Data availability
All relevant data are within this paper and its Supporting Information files. The datasets used or analyzed during this study are available from the corresponding authors upon reasonable request.
References
Martin, S. S. et al. 2025 heart disease and stroke statistics: a report of US and global data from the American heart association. Circulation https://doi.org/10.1161/cir.0000000000001303 (2025).
Beghini, A. et al. 2024 update in heart failure. ESC Heart Fail. https://doi.org/10.1002/ehf2.14857 (2024).
World Health Organization. WHO Cardiovascular diseases (CVDs). (2025). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
Luan, Y. et al. Cardiac cell senescence: molecular mechanisms, key proteins and therapeutic targets. Cell. Death Discovery 10, (2024).
Grootaert, M. O. J. Cell senescence in cardiometabolic diseases. Npj Aging 10, (2024).
Evangelou, K. et al. Cellular senescence and cardiovascular diseases: moving to the heart of the problem. Physiol. Rev. 103, 609–647 (2022).
González-Gualda, E., Baker, A. G., Fruk, L. & Muñoz‐Espín D. A guide to assessing cellular senescencein Vitroandin vivo. FEBS J. 288, 56–80 (2020).
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Kumari, R. & Jat, P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front. Cell. Dev. Biology 9, (2021).
Shimizu, I. & Minamino, T. Cellular senescence in cardiac diseases. J. Cardiol. 74, 313–319 (2019).
Zhao, S., Zhang, Y., Zhao, Y. & Lu, X. Cellular senescence as a key player in chronic heart failure pathogenesis: unraveling mechanisms and therapeutic opportunities. Prog. Biophys. Mol. Biol. https://doi.org/10.1016/j.pbiomolbio.2025.02.002 (2025).
Stojanović, S. D., Thum, T. & Bauersachs, J. Anti-senescence therapies: a new concept to address cardiovascular disease. Cardiovascular. Res. https://doi.org/10.1093/cvr/cvaf030 (2025).
Suda, M., Katsuumi, G., Tchkonia, T., Kirkland, J. L. & Minamino, T. Potential clinical implications of senotherapies for cardiovascular disease. Circ. J. 88, 277–284 (2023).
Xia, W. et al. Depletion of SASP senescent cardiomyocytes with senolytic drugs confers therapeutic effects in doxorubicin-related cardiotoxicity. FEBS J. https://doi.org/10.1111/febs.17164 (2024).
Scalise, M. et al. Senolytics rejuvenate aging cardiomyopathy in human cardiac organoids. Mech. Ageing Dev. 112007 https://doi.org/10.1016/j.mad.2024.112007 (2024).
Salerno, N. et al. Pharmacological clearance of senescent cells improves cardiac remodeling and function after myocardial infarction in female aged mice. Mech. Ageing Dev. 208, 111740 (2022).
Yang, B. et al. Ruxolitinib-based senomorphic therapy mitigates cardiomyocyte senescence in septic cardiomyopathy by inhibiting the JAK2/STAT3 signaling pathway. Int. J. Biol. Sci. 20, 4314–4340 (2024).
Monsen, V. T. & Attramadal, H. Structural insights into regulation of CCN protein activities and functions. J. Cell. Communication Signal. 17, 371–390 (2023).
Chen, C. C. & Lau, L. F. Functions and mechanisms of action of CCN matricellular proteins. Int. J. Biochem. Cell Biol. 41, 771–783 (2008).
Jeong, D. et al. Matricellular protein CCN5 reverses established cardiac fibrosis. J. Am. Coll. Cardiol. 67, 1556–1568 (2016).
Yoon, P. O. et al. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J. Mol. Cell. Cardiol. 49, 294–303 (2010).
Zhang, L., Li, Y., Liang, C. & Yang, W. CCN5 overexpression inhibits profibrotic phenotypes via the PI3K/Akt signaling pathway in lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis and in an in vivo model of lung fibrosis. Int. J. Mol. Med. 33, 478–486 (2013).
Feng, T. et al. CCN1-Induced cellular senescence promotes heart regeneration. Circulation 139, 2495–2498 (2019).
Jun, J. I. & Lau, L. F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 12, 676–685 (2010).
Jun, J. I. & Lau, L. F. CCN2 induces cellular senescence in fibroblasts. J. Cell. Communication Signal. 11, 15–23 (2016).
Tejedor-Santamaria, L. et al. CCN2 activates cellular senescence leading to kidney fibrosis in folic Acid-Induced experimental nephropathy. Int. J. Mol. Sci. 26, 4401 (2025).
Von Hoff, D. D. Risk factors for doxorubicin-lnduced congestive heart failure. Ann. Intern. Med. 91, 710 (1979).
Maejima, Y., Adachi, S., Ito, H., Hirao, K. & Isobe, M. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell. 7, 125–136 (2007).
Spallarossa, P. et al. Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. AJP Heart Circ. Physiol. 297, H2169–H2181 (2009).
Tang, X., Li, P. H. & Chen, H. Z. Cardiomyocyte senescence and cellular communications within myocardial microenvironments. Front. Endocrinol. 11, (2020).
Lewis-McDougall, F. C. et al. Aged‐senescent cells contribute to impaired heart regeneration. Aging Cell. 18, (2019).
Kato, K. et al. Impaired myofibroblast dedifferentiation contributes to nonresolving fibrosis in aging. Am. J. Respir. Cell Mol. Biol. 62, 633–644 (2020).
Magadum, A. et al. Specific modified mRNA translation system. Circulation 142, 2485–2488 (2020).
Song, M. H. et al. Modified mRNA-Mediated CCN5 gene transfer ameliorates cardiac dysfunction and fibrosis without adverse structural remodeling. Int. J. Mol. Sci. 25, 6262 (2024).
Feng, M. et al. Inhibition of cellular communication network factor 1 (CCN1)-driven senescence slows down cartilage inflammaging and osteoarthritis. Bone 139, 115522 (2020).
Kuwahara, M. et al. CCN3 (NOV) drives degradative changes in aging articular cartilage. Int. J. Mol. Sci. 21, 7556 (2020).
Fujita, M., Sasada, M., Iyoda, T. & Fukai, F. Involvement of matricellular proteins in cellular senescence: potential therapeutic targets for Age-Related diseases. Int. J. Mol. Sci. 25, 6591 (2024).
Im, S., Song, M. H., Elangovan, M., Woo, K. M. & Park, W. J. The matricellular protein CCN5 prevents anti-VEGF drug-induced epithelial-mesenchymal transition of retinal pigment epithelium. Sci. Rep. 14, (2024).
Yoon, A. et al. The matricellular protein CCN5 inhibits fibrotic deformation of retinal pigment epithelium. PLoS One. 13, e0208897. https://doi.org/10.1371/journal.pone.0208897 (2018).
Im, S. et al. Suppression of choroidal neovascularization and epithelial-mesenchymal transition in retinal pigmented epithelium by adeno-associated virus-mediated overexpression of CCN5 in mice. PLoS One. 17, e0269937. https://doi.org/10.1371/journal.pone.0269937 (2022).
Patricelli, C., Lehmann, P., Oxford, J. T. & Pu, X. Doxorubicin-induced modulation of TGF-β signaling cascade in mouse fibroblasts: insights into cardiotoxicity mechanisms. Sci. Rep. 13, (2023).
Song, M. H. et al. The TSP-1 domain of the matricellular protein CCN5 is essential for its nuclear localization and anti-fibrotic function. PLoS ONE. 17, e0267629 (2022).
Acknowledgements
We thank Dr. YS. Oh (Central Research Facility of Gwangju Institute of Science and Technology) for technical assistance of the confocal microscopy. Figures 1 A, 2 A, 3 A, 4 A, 5 A and 5D were created with BioRender.com. (https://www.biorender.com, https://BioRender.com/w1 × 0kdx). During the preparation of this manuscript, the authors used ChatGPT (version 5.0) to assist with proofreading and improving the readability of the manuscript. All content was reviewed and edited by the authors, who take full responsibility for the final version of the publication.
Funding
This study was supported by funding from the Korea–US Collaborative Research Fund (RS-2024-00466906), the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM5362521) and National Research Council of Science & Technology (NST) grant by the Korea government (MSIT) (GTL24022-000).
Author information
Authors and Affiliations
Contributions
Conceptualization, S.P.J. and W.J.P.; methodology, Y.J.J. and M.Y.L.; validation, S.B.K., Y.J.J. and M.Y.L.; formal analysis, Y.J.J.; investigation, Y.J.J.; data curation, S.B.K. and S.P.J.; writing—original draft preparation, Y.J.J.; writing—review and editing, Y.J.J., S.P.J. and W.J.P.; supervision, S.P.J. and W.J.P.; Resources, T.H.K. and D.T.J.; project administration, W.J.P.; funding acquisition, W.J.P.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jo, Y., Lee, M., Kim, S.B. et al. The matricellular protein CCN5 (WISP2) inhibits cellular senescence in cardiac myoblasts and fibroblasts. Sci Rep (2026). https://doi.org/10.1038/s41598-026-40206-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-40206-1