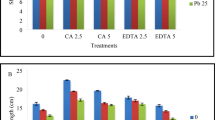
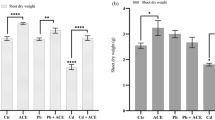
Abstract
Contamination of heavy metals in agricultural soils, particularly with lead (Pb), poses a severe hazard to ecosystems, crop production, and food safety. Although citric acid has been proposed as a potential detoxifying agent, its dose-dependent effects on Pb-stressed tomato plants under controlled conditions are not well understood. A hydroponic experiment was conducted at Khulna Agricultural University, Bangladesh, from January to March 2023 to assess the impact of CA application on tomato seedlings under Pb stress. However, Pb stress significantly impaired plant growth, water content, photosynthetic pigments, and ionic contents (Ca2+, Mg2+) while increasing water loss, electrolyte leakage, and Pb2+ content compared to the control condition. In this study, the CA treatment, particularly HM2 + CA2 treatment, showed the most significant improvements compared to HM2 stress only. Results showed that HM2 + CA2 significantly boosted seedling growth compared to HM2 stress only by increasing root and shoot biomass, plant height, root number, and root volume. It also significantly improved relative water content, total chlorophyll, beta-carotene, carotenoids, and Ca2+ and Mg2+ accumulation in roots and leaves. Additionally, HM2 + CA2 significantly reduced water loss, electrolyte leakage, and Pb2+ content in roots and leaves compared to HM2 stress only, demonstrating its strong protective effects under heavy metal stress. Hierarchical clustering, PCA, and correlation analyses showed clear separation between Pb-only and CA-treated plants, with the latter displaying improved growth, pigment levels, nutrient status, and water balance, especially under the higher CA dose. These results highlight citric acid’s strong capacity to mitigate Pb stress. However, the study’s hydroponic setup and elevated Pb levels represent limitations that necessitate validation under field conditions, and while higher CA concentrations (CA2) were effective, excessive CA use may pose risks of phytotoxicity or nutrient imbalance, highlighting the need for dose optimization. Overall, the findings support organic acids as promising tools for managing heavy metal contamination.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author (S.I.), upon reasonable request.
References
Sarker, P. et al. Indole-3-acetic acid (IAA) assisted phyto-extraction potential of Ipomoea aquatica exposed to lead (Pb) stress. J. Agric. Crops 9, 376–383. https://doi.org/10.32861/jac.93.376.383 (2023).
Zainab, N. et al. PGPR-mediated plant growth attributes and metal extraction ability of Sesbania sesban L. in: Industrially contaminated soils. Agron 11, 1820. https://doi.org/10.3390/agronomy11091820 (2021).
Sarker, P. et al. Exogenous application of synthetic auxin (2,4-dichlorophenoxyacetic acid) impacts on growth, yield, and nutritional parameters of lentil (Lens culinaris M.). J. Plant Nutr. 46, 4559–4572. https://doi.org/10.1080/01904167.2023.2238755 (2023).
Rahman, Z. & Singh, V. P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 191, 1–21. https://doi.org/10.1007/s10661-019-7528-7 (2019).
Khatun, J., Intekhab, A. & Dhak, D. Effect of uncontrolled fertilization and heavy metal toxicity associated with arsenic (As), lead (Pb) and cadmium (Cd), and possible remediation. Toxicology 477, 153274. https://doi.org/10.1016/j.tox.2022.153274 (2022).
Briseño-Bugarín, J. et al. Lead (Pb) pollution in soil: A systematic review and meta-analysis of contamination grade and health risk in Mexico. Environments 11, 43. https://doi.org/10.3390/environments11030043 (2024).
Zulfiqar, U. et al. Lead toxicity in plants: Impacts and remediation. J. Environ. Manage. 250, 109557. https://doi.org/10.1016/j.jenvman.2019.109557 (2019).
Hasanuzzaman, M. et al. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 13, 203–212. https://doi.org/10.1080/17429145.2018.1458913 (2018).
Ghorbani, A. et al. Melatonin-mediated nitric oxide signaling enhances adaptation of tomato plants to aluminum stress. S. Afr. J. Bot. 162, 443–450. https://doi.org/10.1016/j.sajb.2023.09.031 (2023).
Nas, F. S. & Ali, M. The effect of lead on plants in terms of growing and biochemical parameters: A review. MOJ Ecology & Environmental Sciences 3, 265–268. https://doi.org/10.15406/mojes.2018.03.00098 (2018).
Kumar, A. et al. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 17, 2179. https://doi.org/10.3390/ijerph17072179 (2020).
Helmi, A. & Mohamed, H. I. Biochemical and ultrastructural changes in some tomato cultivars after infestation by Aphis gossypii Glover (Hemiptera: Aphididae) in Qalyubiyah, Egypt. Gesunde Pflanzen 68, 41–50 (2016).
Piotto, F. A. et al. Estimating tomato tolerance to heavy metal toxicity: Cadmium as study case. Environ. Sci. Pollut. Res. 25, 27535–27544. https://doi.org/10.1007/s11356-018-2778-4 (2018).
Akinci, I. E., Akinci, S. & Yilmaz, K. Response of tomato (Solanum lycopersicum L.) to lead toxicity: Growth, element uptake, chlorophyll and water content. Afr. J. Agric. Res. 5, 416–423 (2010).
Badiaa, O., Yssaad, H. A. R. & Topcuoglu, B. Effect of heavy metals (copper and zinc) on proline, polyphenols and flavonoids content of tomato (Lycopersicon esculentum Mill.). Plant Arch. 20, 09725210 (2020).
Obi-Iyeke, G. & Ogbara, E. Effects of lead on the growth of tomato (Lycopersicon esculentum Miller.). FUDMA J. Sci. 6, 191–199. https://doi.org/10.33003/fjs-2022-0601-867 (2022).
Jadid, N. et al. Genetic diversity and growth responses of Indonesian tomato (Solanum lycopersicum L.) genotypes under lead stress. Sci. Prog. 105, 368504221122364. https://doi.org/10.1177/00368504221122364 (2022).
Bali, S. et al. Jasmonic acid application triggers detoxification of lead (Pb) toxicity in tomato through the modifications of secondary metabolites and gene expression. Chemosphere 235, 734–748. https://doi.org/10.1016/j.chemosphere.2019.06.188 (2019).
Ma, J. et al. Impact of foliar application of syringic acid on tomato (Solanum lycopersicum L.) under heavy metal stress—Insights into nutrient uptake, redox homeostasis, oxidative stress, and antioxidant defense. Front. Plant Sci. 13, 950120. https://doi.org/10.3389/fpls.2022.950120 (2022).
Afzaal, Z. et al. Lead induced modulation in growth, chlorophyll pigment, nutrient uptake, antioxidant enzyme regulation, gene expression and fruit quality in two tomato cultivars. Int. J. Agric. Biol. 24, 1732–1744 (2020).
Saleem, M. H. et al. Individual and combined application of EDTA and citric acid assisted phytoextraction of copper using jute (Corchorus capsularis L.) seedlings. Environ. Technol. Innov. 19, 100895. https://doi.org/10.1016/j.eti.2020.100895 (2020).
Tahjib-Ul-Arif, M. et al. Citric acid-mediated abiotic stress tolerance in plants. Int. J. Mol. Sci. 22, 7235. https://doi.org/10.3390/ijms22137235 (2021).
Wang, S., Dong, Q. & Wang, Z. Differential effects of citric acid on cadmium uptake and accumulation between tall fescue and Kentucky bluegrass. Ecotoxicol. Environ. Saf. 145, 200–206. https://doi.org/10.1016/j.ecoenv.2017.07.034 (2017).
Kaur, R. et al. Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd (II) toxicity. Ecotoxicol. Environ. Saf. 145, 466–475. https://doi.org/10.1016/j.ecoenv.2017.07.067 (2017).
Sebastian, A. & Prasad, M. N. V. Exogenous citrate and malate alleviate cadmium stress in Oryza sativa L.: Probing role of cadmium localization and iron nutrition. Ecotoxicology and Environmental Safety 166, 215–222. https://doi.org/10.1016/j.ecoenv.2018.09.084 (2018).
Zhang, S. et al. Effects of exogenous organic acids on Cd tolerance mechanism of Salix variegata Franch. under Cd stress. Front. Plant Sci. 11, 594352. https://doi.org/10.3389/fpls.2020.594352 (2020).
Anwer, S., Ashraf, Y. M., Hussain, M., Ashraf, M. & Jamil, A. Citric acid mediated phytoextraction of cadmium by maize (Zea Mays L). Pak. J. Bot. 44, 1831–1836 (2012).
Ehsan, S. et al. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicology and Environmental Safety 106, 164–172. https://doi.org/10.1016/j.ecoenv.2014.03.007 (2014).
Zaheer, I. E. et al. Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicology and Environmental Safety 120, 310–317. https://doi.org/10.1016/j.ecoenv.2015.06.020 (2015).
Mallhi, Z. I. et al. Citric acid enhances plant growth, photosynthesis, and phytoextraction of lead by alleviating the oxidative stress in castor beans. Plants 8, 525. https://doi.org/10.3390/plants8110525 (2019).
Shakoor, M. B. et al. Citric acid improves lead (Pb) phytoextraction in Brassica napus L. by mitigating Pb-induced morphological and biochemical damages. Ecotoxicology and Environmental Safety 109, 38–47. https://doi.org/10.1016/j.ecoenv.2014.07.033 (2014).
Al Mahmud, J., Hasanuzzaman, M., Nahar, K., Bhuyan, M. B. & Fujita, M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 147, 990–1001. https://doi.org/10.1016/j.ecoenv.2017.09.045 (2018).
Kumar, A., Pal, L. & Agrawal, V. Glutathione and citric acid modulates lead-and arsenic-induced phytotoxicity and genotoxicity responses in two cultivars of Solanum lycopersicum L. Acta Physiol. Plant. 39, 1–12. https://doi.org/10.1007/s11738-017-2448-z (2017).
Chen, Y. X. et al. The role of citric acid on the phytoremediation of heavy metal contaminated soil. Chemosphere 50, 807–811. https://doi.org/10.1016/S0045-6535(02)00223-0 (2003).
Cheng, S. F., Huang, C. Y. & Tu, Y. T. Remediation of soils contaminated with chromium using citric and hydrochloric acids: The role of chromium fractionation in chromium leaching. Environ. Technol. 32, 879–889. https://doi.org/10.1080/09593330.2010.517218 (2011).
Ke, X. et al. Removal of Cd, Pb, Zn, Cu in smelter soil by citric acid leaching. Chemosphere 255, 126690. https://doi.org/10.1016/j.chemosphere.2020.126690 (2020).
Kanwal, U. et al. Phytoextraction of lead using a hedge plant [Alternanthera bettzickiana (Regel) G. Nicholson]: Physiological and biochemical alterations through bioresource management. Sustainability 13, 5074. https://doi.org/10.3390/su13095074 (2021).
Tian, X. Y. et al. Physiological and molecular advances in magnesium nutrition of plants. Plant Soil 468, 1–2. https://doi.org/10.1007/s11104-021-05139-w (2021).
Jing, T. et al. Role of calcium nutrition in plant physiology: Advances in research and insights into acidic soil conditions—a comprehensive review. Plant Physiol. Biochem. 210, 108602. https://doi.org/10.1016/j.plaphy.2024.108602 (2024).
Amir, W. et al. Accumulation potential and tolerance response of Typha latifolia L. under citric acid assisted phytoextraction of lead and mercury. Chemosphere 257, 127247. https://doi.org/10.1016/j.chemosphere.2020.127247 (2020).
Imran, S. et al. Seed priming and exogenous application of citric acid enhance seedling growth and photosynthetic pigments and mitigate oxidative damage of soybean (Glycine max) under salt stress. Arch. Biol. Sci. 75, 407–418. https://doi.org/10.2298/ABS230804033I (2023).
Mostofa, M. G. & Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicol. 22, 959–973. https://doi.org/10.1007/s10646-013-1073-x (2013).
Chakrobortty, J., Imran, S., Mahamud, M. A., Sarker, P. & Paul, N. C. Effect of citric acid (CA) priming and exogenous application on germination and early seedling growth of okra (Abelmoschus esculentus L.) plants under salinity stress condition. Arch. Agric. Environ. Sci. 7, 318–326. https://doi.org/10.26832/24566632.2022.070303 (2022).
Tania, S. S. et al. Alleviation of salt-inhibited germination and seedling growth of kidney bean by seed priming and exogenous application of salicylic acid (SA) and hydrogen peroxide (H₂O₂). Seeds 1, 87–98. https://doi.org/10.3390/seeds1020008 (2022).
Hossain, M. M. et al. Citric acid and hydro-priming and exogenous application alleviate salt-inhibited seed germination and seedling growth of chilli (Capsicum annuum L.). J. Agric. Crops 9, 495–502. https://doi.org/10.32861/jac.94.495.502 (2023).
Rahman, S., Shaheen, M. S., Rahman, M. & Malik, T. A. Evaluation of excised leaf water loss and relative water content as screening techniques for breeding drought resistant wheat. Pak. J. Biol. Sci. 3, 663–665. https://doi.org/10.3923/pjbs.2000.663.665 (2000).
Diao, Q., Song, Y., Shi, D. & Qi, H. Interaction of polyamines, abscisic acid, nitric oxide, and hydrogen peroxide under chilling stress in tomato (Lycopersicon esculentum Mill.) seedlings. Front. Plant Sci. 8, 203. https://doi.org/10.3389/fpls.2017.00203 (2017).
Hniličková, H., Hnilička, F., Orsák, M. & Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na + and K+ content in selected plant species. Plant. Soil. Environ. 65, 90–96. https://doi.org/10.17221/620/2018-PSE (2019).
Mahamud, M. A., Chowdhury, M. A., Rahim, M. A. & Mohiuddin, K. M. Mineral nutrient contents of some potato accessions of USA and Bangladesh. J. Bangladesh Agric. Univ. 13, 207–214. https://doi.org/10.22004/ag.econ.235282 (2015).
Udo, E. J., Ibia, T. O., Ogunwale, J. A., Ano, A. O. & Esu, I. E. Manual of soil, plant, and water analyses (Sibon Books, 2009).
APHA. Standard Methods for the Examination of Water and Wastewater, 22nd edn. American Public Health Association, (2012).
Mukaka, M. M. A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 24, 69–71 (2012).
Piotrowska, A., Bajguz, A., Godlewska-Żyłkiewicz, B., Czerpak, R. & Kamińska, M. Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lemnaceae). Environ. Exp. Bot. 66, 507–513. https://doi.org/10.1016/j.envexpbot.2009.03.019 (2009).
Singh, R. et al. Lead bioaccumulation potential of an aquatic macrophyte Najas indica are related to antioxidant system. Bioresource Technol. 101, 3025–3032. https://doi.org/10.1016/j.biortech.2009.12.031 (2010).
Okant, M. & Kaya, C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Res. 26, 11864–11874. https://doi.org/10.1007/s11356-019-04517-3 (2019).
Gopal, R. & Rizvi, A. H. Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere 70, 1539–1544. https://doi.org/10.1016/j.chemosphere.2007.08.043 (2008).
Islam, E. et al. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 147, 806–816. https://doi.org/10.1016/j.jhazmat.2007.01.117 (2007).
Arias, J. A. et al. Effects of Glomus deserticola inoculation on Prosopis: Enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM techniques. Environ. Exp. Bot. 68, 139–148. https://doi.org/10.1016/j.envexpbot.2009.08.009 (2010).
Jiang, W. & Liu, D. Pb-induced cellular defense system in the root meristematic cells of Allium sativum L. BMC Plant Biol. 10, 40. https://doi.org/10.1186/1471-2229-10-40 (2010).
Afshan, S. et al. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res. 22, 11679–11689. https://doi.org/10.1007/s11356-015-4396-8 (2015).
Gao, Y. et al. Improvement of phytoextraction and antioxidative defense in Solanum nigrum L. under cadmium stress by application of cadmium-resistant strain and citric acid. J. Hazard. Mater. 181, 771–777. https://doi.org/10.1016/j.jhazmat.2010.05.080 (2010).
Song, J. et al. Exogenous oxalic acid and citric acid improve lead (Pb) tolerance of Larix olgensis A. Henry seedlings. Forests 9, 510. https://doi.org/10.3390/f9090510 (2018).
Kim, D. J., Park, B. C., Ahn, B. K. & Lee, J. H. Thallium uptake and translocation in barley and sunflower grown in hydroponic conditions. Int. J. Environ. Res. 10, 575–582. https://doi.org/10.22059/ijer.2016.59686 (2016).
Rodriguez, E. et al. Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol. Biochem. 53, 94–100. https://doi.org/10.1016/j.plaphy.2012.01.013 (2012).
Gupta, M. et al. Lead toxicity in plants: Mechanistic insights into toxicity, physiological responses of plants and mitigation strategies. Plant Signal. Behav. 19, 2365576. https://doi.org/10.1080/15592324.2024.2365576 (2024).
Jin, X. et al. Effect of citric acid seed priming on the growth and physiological characteristics of tomato seedlings under low phosphorus stress. Chin. J. Eco-Agric. 29, 1159–1170. https://doi.org/10.13930/j.cnki.cjea.200953 (2021).
Ali, S. et al. Combined application of citric acid and Cr resistant microbes improved castor bean growth and photosynthesis while it alleviated Cr toxicity by reducing Cr+ 6 to Cr3+. Microorganisms 9, 2499. https://doi.org/10.3390/microorganisms9122499 (2021).
Ali, B. et al. 5-Aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity, and ultrastructural changes under cadmium stress in Brassica napus L. J. Plant Growth Regul. 32, 604–614. https://doi.org/10.1007/s00344-013-9328-6 (2013).
Farid, M. et al. Citric acid assisted phytoextraction of chromium by sunflower; Morpho-physiological and biochemical alterations in plants. Ecotoxicol. Environ. Saf. 145, 90–102. https://doi.org/10.1016/j.ecoenv.2017.07.016 (2017).
Han, Y., Zhang, L., Gu, J., Zhao, J. & Fu, J. Citric acid and EDTA on the growth, photosynthetic properties and heavy metal accumulation of Iris halophila Pall. cultivated in Pb mine tailings. Int. Biodeterior. Biodegrad. 128, 15–21. https://doi.org/10.1016/j.ibiod.2016.05.011 (2018).
Wang, H. Y., Tong, H. Y., Huang, S. Z. & Yuan, H. Y. Effects of citric acid and oxalic acid on the growth and physiology of Iris lactea var. chinensis under Pb stress. Chin. J. Ecol. 29, 1340–1346 (2010).
Ma, J. et al. Chemical and mechanical coating of sulfur on baby corn biochar and their role in soil Pb availability, uptake, and growth of tomato under Pb contamination. Environ. Pollut. 338, 122654. https://doi.org/10.1016/j.envpol.2023.122654 (2023).
Perez-Labrada, F., Benavides-Mendoza, A., Valdez-Aguilar, L. A. & Robledo-Torres, V. Citric acid in the nutrient solution increases the mineral absorption in potted tomato grown in calcareous soil. Pak. J. Bot. 48, 67–74 (2016).
Rhaman, M. S. et al. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 10, 37. https://doi.org/10.3390/plants10010037 (2020).
Hajaji, A. N., Maaroufi-Dguimi, H. & Ammari, Y. Exogenous application of citric acid mitigates salt-induced oxidative stress in Moringa oleifera seedlings. J. Umm Al-Qura Univ. Appl. Sci. https://doi.org/10.1007/s43994-024-00169-3 (2024).
Author information
Authors and Affiliations
Contributions
S.I. designed the experiment; M.A.M., S.I., P.S., J.C., and N.C.P. performed the experiment; S.I. and M.A.R. conducted pigments and ionic estimation; S.I. performed the formal analysis; M.A.M., S.I., P.S., J.C., N.C.P., M.J.S., and M.A.R. wrote the original draft of the manuscript; S.I., S.H. and M.R. revised and corrected the manuscript. All the authors discussed the results, contributed to the final manuscript, and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
“The experiments did not involve endangered or protected species. The plant material was obtained from the Khulna Agricultural University, Khulna, Bangladesh. No special permissions were necessary to collect samples. Otherwise, the plant materials used and collected in the study comply with Bangladeshi guidelines and legislation”.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mahamud, M.A., Imran, S., Sarker, P. et al. Foliar application of citric acid alleviates lead toxicity and enhances physiological resilience in tomato seedlings. Sci Rep (2026). https://doi.org/10.1038/s41598-026-40466-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-40466-x