Abstract
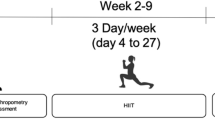
Overweight and obesity are major global health concerns linked to chronic diseases. This study compared the effects of high-intensity functional training (HIFT) and resistance training (RT) on metabolic, inflammatory, and physical markers in overweight men. Thirty-four overweight men (31.91 ± 2.44 years, BMI 27.78 ± 1.47 kg/m2) were assigned to the HIFT (n = 13), RT (n = 10), and control (n = 11) groups. The HIFT included four sets of 30-second exercises at 30% 1RM, whereas the RT involved three sets of 12 repetitions at 70% 1RM. Both interventions were performed thrice weekly for eight weeks. Blood samples were collected before and after training to assess the levels of inflammatory markers (IL-4, γ-IFN, MMP-9 and TLR4) and metabolic markers (FBS, LDL, HDL, triglycerides and cholesterol). Body composition and performance were evaluated. No significant differences in inflammatory markers were detected between the groups. HIFT and RT significantly reduced fasting blood sugar (p < 0.05). RT lowered total cholesterol, whereas triglycerides decreased in both groups. Skeletal muscle mass increased significantly. Compared with RT, HIFT led to greater body fat reduction, although both improved from baseline. Both groups had increased 1RM bench press strength. Compared with RT and the control, HIFT significantly increased VO2max, whereas RT improved VO2max compared with the control. HIFT is a promising approach for managing overweight and obesity, particularly for enhancing cardiovascular fitness and body composition.
Trial registration IRCT.ir, IRCT20170724035269N2, registered on 27/05/2025. This study was retrospectively registered.
Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to participant privacy and confidentiality considerations but are available from the corresponding author on reasonable request. All shared data will be fully de-identified to protect participant privacy.
References
Tabarés Seisdedos, R. Health effects of overweight and obesity in 195 countries over 25 years (2017).
Rogero, M. M. & Calder, P. C. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients 10(4), 432 (2018).
Ayatollahi, S. & Ghoreshizadeh, Z. Prevalence of obesity and overweight among adults in Iran. Obes. Rev. 11(5), 335–337 (2010).
Campos-Bayardo, T. I. et al. The role of TLRs in obesity and its related metabolic disorders. Int. J. Mol. Sci. 26(5), 2229 (2025).
Gleeson, M. et al. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11(9), 607–615 (2011).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542(7640), 177–185 (2017).
Shi, H. et al. TLR4 links innate immunity and fatty acid–induced insulin resistance. J. Clin. Investig. 116(11), 3015–3025 (2006).
Orr, J. S. et al. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes 61(11), 2718–2727 (2012).
Ahmad, R. et al. Elevated expression of the toll like receptors 2 and 4 in obese individuals: Its significance for obesity-induced inflammation. J. Inflamm. 9(1), 48 (2012).
Langjahr, P. et al. Metalloproteinase-dependent TLR2 ectodomain shedding is involved in soluble toll-like receptor 2 (sTLR2) production. PloS One 9(12), e104624 (2014).
Jaoude, J. & Koh, Y. Matrix metalloproteinases in exercise and obesity. Vasc. Health Risk Manag. 287, 95 (2016).
Wensveen, F. M., Valentić, S., Šestan, M., Turk Wensveen, T. & Polić, B. The big Bang in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur. J. Immunol. 45(9), 2446–2456 (2015).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 117(1), 175–184 (2007).
Soltani, N., Marandi, S. M., Kazemi, M. & Esmaeil, N. Combined all-extremity high-intensity interval training regulates immunometabolic responses through toll-like receptor 4 adaptors and A20 downregulation in obese young females. Obes. Facts 13(3), 415–431 (2020).
Filipovic, T. et al. Research article effects of 12-week exercise program on enzyme activity of serum matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in female patients with postmenopausal osteoporosis: A randomized control study (2020).
Lee, M-G., Park, K-S., Kim, D-U., Choi, S-M. & Kim, H-J. Effects of high-intensity exercise training on body composition, abdominal fat loss, and cardiorespiratory fitness in middle-aged Korean females. Appl. Physiol. Nutr. Metab. 37(6), 1019–1027 (2012).
Nikseresht, M. Comparison of serum cytokine levels in men who are obese or men who are lean: Effects of nonlinear periodized resistance training and obesity. J. Strength Cond. Res. 32(6), 1787–1795 (2018).
Kim, E. S. et al. Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity 15(12), 3023–3030 (2007).
Atashak, S. et al. High-intensity interval training improves lipocalin-2 and omentin-1 levels in men with obesity. Int. J. Sports Med. 43(04), 328–335 (2022).
Tayebi, S. M., Ghanbari-Niaki, A., Saeidi, A. & Hackney, A. C. Exercise training, neuregulin 4 and obesity. Ann. Appl. Sport Sci. 5(2), 1 (2017).
Saeidi, A. et al. Astaxanthin supplemented with high-intensity functional training decreases adipokines levels and cardiovascular risk factors in men with obesity. Nutrients 15(2), 286 (2023).
Supriya, R. et al. Spirulina supplementation with high-intensity interval training decreases adipokines levels and cardiovascular risk factors in men with obesity. Nutrients 15(23), 4891 (2023).
Feito, Y., Heinrich, K. M., Butcher, S. J. & Poston, W. S. C. High-intensity functional training (HIFT): Definition and research implications for improved fitness. Sports 6(3), 76 (2018).
Murawska-Cialowicz, E., Wojna, J. & Zuwala-Jagiello, J. Crossfit training changes brain-derived neurotrophic factor and Irisin levels at rest, after wingate and progressive tests, and improves aerobic capacity and body composition of young physically active men and women. J. Physiol. Pharmacol. 66(6), 811–821 (2015).
NSCA Science of Strength & Conditioning. Essentials of strength training and conditioning: Human kinetics (2021).
McDougle, J. M., Mangine, G. T., Townsend, J. R., Jajtner, A. R. & Feito, Y. Acute physiological outcomes of high-intensity functional training: A scoping review. PeerJ 11, e14493 (2023).
Santos, D. A. T. et al. Comparison of physiological and psychobiological acute responses between high intensity functional training and high intensity continuous training. Sports Med. Health Sci. 7(1), 68–76 (2025).
Borga, M. et al. Advanced body composition assessment: From body mass index to body composition profiling. J. Invest. Med. 66(5), 1–9 (2018).
Zhang, F. L. et al. Strong association of waist circumference (WC), body mass index (BMI), waist-to-height ratio (WHtR), and waist-to-hip ratio (WHR) with diabetes: A population-based cross-sectional study in Jilin Province, China. J. Diabetes Res. 2021, 8812431 (2021).
Riebe, D., Ehrman, J., Liguori, G. & Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription 226–363 (2018).
Buttar, K., Scholar, Saboo, N. & Kacker, S. A review: Maximal oxygen uptake (VO2 max) and its estimation methods. Int. J. Phys. Educ. Sports Health 6(6), 24–32 (2019).
Pournot, H. et al. Time-course of changes in inflammatory response after whole-body cryotherapy multi exposures following severe exercise. PloS One 6(7), e22748 (2011).
Soltani, N., Marandi, S. M., Kazemi, M. & Esmaeil, N. Meta-inflammatory state and insulin resistance can improve after 10 weeks of combined all-extremity high-intensity interval training in sedentary overweight/obese females: A quasi-experimental study. J. Diabetes Metab. Disord. 19(2), 717–726 (2020).
Bastarache, J. A. et al. Accuracy and reproducibility of a multiplex immunoassay platform: A validation study. J. Immunol. Methods 367(1–2), 33–39 (2011).
Leng, S. X. et al. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 63(8), 879–884 (2008).
Favere, K. et al. A systematic literature review on the effects of exercise on human toll-like receptor expression. Exerc. Immunol. Rev. 27, 84–124 (2021).
Church, T. S. et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: A randomized controlled trial. Jama 304(20), 2253–2262 (2010).
Jelleyman, C. et al. The effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes. Rev. 16(11), 942–961 (2015).
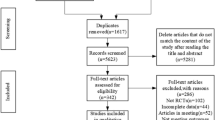
Schulz, K. F., Altman, D. G. & Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 340, c332 (2010).
Robinson, E. et al. Short-term high-intensity interval and moderate-intensity continuous training reduce leukocyte TLR4 in inactive adults at elevated risk of type 2 diabetes. J. Appl. Physiol. 119(5), 508 –516 (2015).
Soltani, N. et al. Assessment of the effect of short-term combined high-intensity interval training on TLR4, NF-κB and IRF3 expression in young overweight and obese girls. Public. Health Genomics 23(1–2), 26–36 (2020).
Reyna, S. M. et al. Short-term exercise training improves insulin sensitivity but does not inhibit inflammatory pathways in immune cells from insulin-resistant subjects. J. Diabetes Res. 2013, 107805 (2013).
Phillips, M. D. et al. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med. Sci. Sports. Exerc. 44(11), 2099–2110 (2012).
Oliveira-Child, M., Leggate, M. & Gleeson, M. Effects of two weeks of high-intensity interval training (HIIT) on monocyte TLR2 and TLR4 expression in high BMI sedentary men. Int. J. Exerc. Sci. 6, 81–90 (2013).
Silveira, L. S. et al. Macrophage polarization: Implications on metabolic diseases and the role of exercise. Crit. Rev. Eukaryot. Gene Expr. 26(2), 115–132 (2016).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14(10), 576–590 (2018).
Rodriguez-Miguelez, P. et al. Role of Toll-like receptor 2 and 4 signaling pathways on the inflammatory response to resistance training in elderly subjects. Age 36(6), 9734 (2014).
Gleeson, M. Immune function in sport and exercise. J. Appl. Physiol. (2007).
Petersen, A. M. W. & Pedersen, B. K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 98(4), 1154–1162 (2005).
Egan, B. & Zierath, J. R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metabol. 17(2), 162–184 (2013).
Handschin, C. & Spiegelman, B. M. The role of exercise and PGC1α in inflammation and chronic disease. Nature 454(7203), 463–469 (2008).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444(7121), 860–867 (2006).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56(7), 1761–1772 (2007).
Jackson, L., Cady, C. T. & Cambier, J. C. TLR4-mediated signaling induces MMP9-dependent cleavage of B cell surface CD23. J. Immunol. 183(4), 2585–2592 (2009).
Lo Presti, R., Hopps, E. & Caimi, G. Gelatinases and physical exercise: A systematic review of evidence from human studies. Medicine 96(37), e8072 (2017).
Filipović, T. et al. Effects of 12-Week exercise program on enzyme activity of serum matrix Metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in female patients with postmenopausal osteoporosis: A randomized control study. Biomed. Res. Int. 2020, 9758289 (2020).
Rullman, E., Olsson, K., Wågsäter, D. & Gustafsson, T. Circulating MMP-9 during exercise in humans. Eur. J. Appl. Physiol. 113(5), 1249–1255 (2013).
Smith, J. S., Bellissimo, G. F. & Amorim, F. T. The physiological responses to volume-matched high-intensity functional training protocols with varied time domains. Front. Physiol. 15, 1511961 (2024).
Prestes, J. et al. The effects of muscle strength responsiveness to periodized resistance training on resistin, leptin, and cytokine in elderly postmenopausal women. J. Strength. Conditioning Res. 32(1), 113–120 (2018).
Kjølhede, T. et al. Acute and chronic cytokine responses to resistance exercise and training in people with multiple sclerosis. Scand. J. Med. Sci. Sports 26(7), 824–834 (2016).
de Souza, D. C. et al. Effects of high-intensity interval and moderate-intensity continuous exercise on inflammatory, leptin, IgA, and lipid peroxidation responses in obese males. Front. Physiol. 9, 567 (2018).
O’rourke, R. et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-γ in inflammation in human adipose tissue. Int. J. Obes. 33(9), 978–990 (2009).
Goossens, G. H. The metabolic phenotype in obesity: Fat mass, body fat distribution, and adipose tissue function. Obes. Facts 10(3), 207–215 (2017).
Kliszczewicz, B., Buresh, R., Bechke, E. & Williamson, C. Metabolic biomarkers following a short and long bout of high-intensity functional training in recreationally trained men (2017).
Yin, M. et al. Is low-volume high-intensity interval training a time-efficient strategy to improve cardiometabolic health and body composition? A meta-analysis. Appl. Physiol. Nutr. Metab. 49(3), 273–292 (2023).
Kapsis, D. P. et al. Changes in body composition and strength after 12 weeks of high-intensity functional training with two different loads in physically active men and women: A randomized controlled study. Sports 10(1), 7 (2022).
Ameur, R. et al. Unlocking the power of synergy: High-intensity functional training and early time-restricted eating for transformative changes in body composition and cardiometabolic health in inactive women with obesity. Plos One 19(5), e0301369 (2024).
Seyed, M. T., Peiman, H., Ayoub, S. & Mohammadreza, F. Intense circuit resistance training along with zataria multifl ora supplementation reduced plasma retinol binding protein-4 and tumor necrosis factor-in postmenopausal females. Jundishapur J. Nat. Pharm. (2018).
Adami, P. et al. Physiological profile comparison between high intensity functional training, endurance and power athletes. Eur. J. Appl. Physiol. 122(2), 531–539 (2022).
Pedersen, B. K. & Febbraio, M. A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. (2008).
Laforgia, J., Withers, R. T. & Gore, C. J. Effects of exercise intensity and duration on the excess post-exercise oxygen consumption. J. Sports Sci. 24(12), 1247–1264 (2006).
Fasshauer, M. & Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 36(7), 461–470 (2015).
Lasevicius, T. et al. Effects of different intensities of resistance training with equated volume load on muscle strength and hypertrophy. Eur. J. Sport Sci. 18(6), 772–780 (2018).
Lacio, M. et al. Effects of resistance training performed with different loads in untrained and trained male adult individuals on maximal strength and muscle hypertrophy: A systematic review. Int. J. Environ. Res. Public Health 18(21), 11237 (2021).
Cosgrove, S. J., Crawford, D. A. & Heinrich, K. M. Multiple fitness improvements found after 6-months of high intensity functional training. Sports 7(9), 203 (2019).
Smith, J. S., Bellissimo, G. F. & Amorim, F. T. The physiological responses to volume-matched high-intensity functional training protocols with varied time domains. Front. Physiol. 15, 1511961 (2025).
Ried-Larsen, M., Aarts, H. M. & Joyner, M. J. Effects of strict prolonged bed rest on cardiorespiratory fitness: Systematic review and meta-analysis. J. Appl. Physiol. 123(4), 790–799 (2017).
Astorino, T. A., Mower, M., Flores, A. & Flannery, M. Cardiometabolic response to high intensity functional training versus rowing-based high intensity interval training. Sports Med Health Sci. (2025).
Tomschi, F., Ransmann, P., Schmidt, A. & Hilberg, T. Exercise induced hypoalgesia after a high intensity functional training: A randomized controlled crossover study. BMC Sports Sci. Med. Rehabil. 16(1), 182 (2024).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16(10), 626–638 (2016).
Acknowledgements
The authors would like to acknowledge Dr. Nooshin Lotfi and the staff of the Immunology Department Laboratoryat Isfahan University of Medical Sciences, Iran, particularly Mrs. Fahimeh Hosseininasab, for their kind andinvaluable support.
Funding
This study did not receive external funding and was not supported by any specific grant from funding agencies in the public, commercial, or not-for-profit.
Author information
Authors and Affiliations
Contributions
F.S.H.M. and S.A. conceptualized and designed the study (this study is based on the master’s thesis of both individuals). F.S.H.M. and N.E. drafted the initial manuscript, conducted the laboratory experiments, and performed the statistical analyses. N.E. also served as an advisor for this project and approved the final manuscript. F.S.H.M. and S.A. conceptualized the study, designed the exercise protocol, and conducted the training sessions. N.E. performed the final editing of the manuscript. F.S. and R.S., as supervisors, oversaw the work and validated the exercise protocol and data. All the authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. This study was reported in accordance with the CONSORT guidelines for randomized controlled trials.
Ethical approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and commenced after obtaining ethical approval from the Institutional Review Board of the Faculty of Sport Sciences, University of Tehran (protocol code IR.UT.SPORT.REC.1401.025, approved in July 2022). Informed consent was obtained from all participants prior to enrollment. Due to limited awareness among local researchers regarding international standards for prospective clinical trial registration and the absence of institutional mandates at the time, the trial was registered retrospectively. Following recognition of international requirements, the trial was formally registered without any modifications to the protocol or dataset in the Iranian Registry of Clinical Trials (IRCT.ir; registration code IRCT20170724035269N2) on May 15, 2025. This retrospective registration was authorized and overseen directly by the Vice-Chancellery for Research of the Faculty of Sport Sciences, University of Tehran, ensuring full transparency and integrity of the research process.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hosseini Moshkenani, F., Abedi, S., Shabkhiz, F. et al. High intensity functional training versus traditional resistance training effects on inflammatory, metabolic, and physical outcomes in overweight men a randomized controlled trial. Sci Rep (2026). https://doi.org/10.1038/s41598-026-40482-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-40482-x