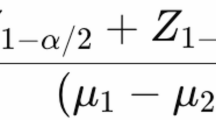Abstract
Endothelial cell dysfunction is reported to occur in polycystic ovary syndrome (PCOS) but it is unclear if this is due to obesity or inherent to PCOS. We hypothesized that in body mass index (BMI)-matched women with obesity, with and without PCOS, endothelial cell dysfunction protein markers would differ due to the inherent pathophysiology of PCOS. 92 women with PCOS and 19 control subjects were identified from a PCOS and control biobank and matched for obesity (BMI ≥ 30 kg/m2). Endothelial dysfunction markers were determined by the Slow Off-rate Modified Aptamer (SOMA)-scan proteomics method. Intercellular adhesion molecule-1 (ICAM1; p < 0.05), tissue plasminogen activator (tPA; p < 0.05), plasminogen activator inhibitor-1 (PAI-1; p < 0.05) and D-dimer (p < 0.003) were significantly elevated in PCOS but did not correlate with either insulin resistance (IR) or hyperandrogenemia. BMI, C-reactive protein (CRP) and sex hormone binding protein (SHBG) did not differ between subjects, but IR and testosterone were increased (p < 0.01) in PCOS, as expected. Endothelial dysfunction is an inherent feature of the pathophysiology found in PCOS, with markers of both endothelial activation/dysfunction (ICAM1 elevation) and coagulation and fibrinolysis (tPA, PAI-1 and D-Dimer elevation) in obese PCOS compared to matched controls. These circulatory proteins are of direct relevance to potential development of cardiovascular disease in PCOS.
Similar content being viewed by others
Data availability
All the data for this study will be made available upon reasonable request to the corresponding author.
References
Norman, R. J., Dewailly, D., Legro, R. S. & Hickey, T. E. Polycystic ovary syndrome. Lancet 370, 685–697 (2007).
Walters, K. A. et al. New Perspectives on the Pathogenesis of PCOS: Neuroendocrine Origins. Trends Endocrinol. Metab. 29, 841–852. https://doi.org/10.1016/j.tem.2018.08.005 (2018).
Christ, J. P. & Cedars, M. I. Current Guidelines for Diagnosing PCOS. Diagnostics (Basel). 13. https://doi.org/10.3390/diagnostics13061113 (2023).
Chang, S. & Dunaif, A. Diagnosis of Polycystic Ovary Syndrome: Which Criteria to Use and When? Endocrinol. Metab. Clin. North. Am. 50, 11–23. https://doi.org/10.1016/j.ecl.2020.10.002 (2021).
Organization, W. H. Polycystic ovary syndrome, (2025).
Solano, M. E., Sander, V. A., Ho, H., Motta, A. B. & Arck, P. C. Systemic inflammation, cellular influx and up-regulation of ovarian VCAM-1 expression in a mouse model of polycystic ovary syndrome (PCOS). J. Reprod. Immunol. 92, 33–44 (2011).
Ollila, M. M., Hoek, A. & Piltonen, T. T. The association between polycystic ovary syndrome and early cardiovascular disease morbidity strengthens. Eur. J. Endocrinol. 189, R4–R5. https://doi.org/10.1093/ejendo/lvad083 (2023).
Sathyapalan, T. & Atkin, S. Mechanisms in endocrinology: recent advances in cardiovascular aspects of polycystic ovary syndrome. Eur. J. Endocrinol. 166, 575–583 (2012).
Guan, C. et al. Polycystic ovary syndrome: a risk-enhancing factor for cardiovascular disease. Fertil. Steril. 117, 924–935 (2022).
Zhang, J., Xu, J. H., Qu, Q. Q. & Zhong, G. Q. Risk of cardiovascular and cerebrovascular events in polycystic ovarian syndrome women: a meta-analysis of cohort studies. Front. Cardiovasc. Med. 7, 552421 (2020).
Wekker, V. et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum. Reprod. Update. 26, 942–960 (2020).
Zhao, L. et al. Polycystic ovary syndrome (PCOS) and the risk of coronary heart disease (CHD): a meta-analysis. Oncotarget 7, 33715 (2016).
Iftikhar, S. et al. Risk of cardiovascular events in patients with polycystic ovary syndrome. Neth. J. Med. 70, 74 (2012).
Tay, C. T. et al. 2023 International Evidence-Based Polycystic Ovary Syndrome Guideline Update: Insights From a Systematic Review and Meta‐Analysis on Elevated Clinical Cardiovascular Disease in Polycystic Ovary Syndrome. J. Am. Heart Assoc. 13, e033572 (2024).
Wan, Z. et al. Risk and incidence of cardiovascular disease associated with polycystic ovary syndrome. Eur. J. Prev. Cardiol. 31, 1560–1570 (2024).
Cena, H., Chiovato, L. & Nappi, R. E. Obesity, polycystic ovary syndrome, and infertility: a new avenue for GLP-1 receptor agonists. J. Clin. Endocrinol. Metabolism. 105, e2695–e2709 (2020).
Puder, J. J. et al. Central fat excess in polycystic ovary syndrome: relation to low-grade inflammation and insulin resistance. J. Clin. Endocrinol. Metabolism. 90, 6014–6021 (2005).
Barber, T. M., Hanson, P., Weickert, M. O. & Franks, S. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin. Med. Insights: Reproductive Health. 13, 1179558119874042 (2019).
Kahal, H. et al. Effect of induced hypoglycemia on inflammation and oxidative stress in type 2 diabetes and control subjects. Sci. Rep. 10, 4750 (2020).
Butler, A. E., Moin, A. S. M., Sathyapalan, T. & Atkin, S. L. Complement dysregulation in obese versus nonobese polycystic ovary syndrome patients. Cells 12, 2022 (2023).
Moin, A. S. M., Sathyapalan, T., Butler, A. E. & Atkin, S. L. Coagulation factor dysregulation in polycystic ovary syndrome is an epiphenomenon of obesity. Clin. Endocrinol. 98, 796–802 (2023).
Vince, R. V., Kirk, R. J., Aye, M. M., Atkin, S. L. & Madden, L. A. Impaired heat shock protein 72 expression in women with polycystic ovary syndrome following a supervised exercise programme. Cell. Stress Chaperones. 25, 73–80 (2020).
Ray, A., Maharana, K. C., Meenakshi, S. & Singh, S. Endothelial dysfunction and its relation in different disorders: Recent update. Health Sci. Rev. 7, 100084 (2023).
Guerby, P. et al. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 40, 101861 (2021).
Oikonomou, E. et al. Coronary artery disease and endothelial dysfunction: novel diagnostic and therapeutic approaches. Curr. Med. Chem. 27, 1052–1080 (2020).
Kajikawa, M. & Higashi, Y. Obesity and endothelial function. Biomedicines 10, 1745 (2022).
Galkina, E. & Ley, K. Vascular Adhesion Molecules in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27, 2292–2301. https://doi.org/10.1161/ATVBAHA.107.149179 (2007).
Saharinen, P., Eklund, L. & Alitalo, K. Therapeutic targeting of the angiopoietin–TIE pathway. Nat. Rev. Drug Discovery. 16, 635–661. https://doi.org/10.1038/nrd.2016.278 (2017).
Okafor, O. N. & Gorog, D. A. Endogenous fibrinolysis: an important mediator of thrombus formation and cardiovascular risk. J. Am. Coll. Cardiol. 65, 1683–1699 (2015).
Deanfield, J. E., Halcox, J. P. & Rabelink, T. J. Endothelial function and dysfunction: testing and clinical relevance. Circulation 115, 1285–1295 (2007).
Szmitko, P. E. et al. New markers of inflammation and endothelial cell activation: Part I. Circulation 108, 1917–1923 (2003).
Brinkworth, G. D., Noakes, M., Moran, L. J., Norman, R. & Clifton, P. M. Flow-mediated dilatation in overweight and obese women with polycystic ovary syndrome. BJOG: Int. J. Obstet. Gynecol. 113, 1308–1314 (2006).
Paradisi, G., Steinberg, H. O., Shepard, M. K., Hook, G. & Baron, A. D. Troglitazone therapy improves endothelial function to near normal levels in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metabolism. 88, 576–580 (2003).
Mather, K. J., Verma, S., Corenblum, B. & Anderson, T. J. Normal endothelial function despite insulin resistance in healthy women with the polycystic ovary syndrome. J. Clin. Endocrinol. Metabolism. 85, 1851–1856 (2000).
Burlá, M. et al. Endothelial dysfunction and pulse wave reflection in women with polycystic ovarian syndrome. Int. J. Cardiovasc. Sci. 32, 3–9 (2019).
Raja-Khan, N. et al. Brachial artery conductance during reactive hyperemia is increased in women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reproductive Biology. 155, 49–53 (2011).
Dawson, A. J. et al. The effect of exenatide on cardiovascular risk markers in women with polycystic ovary syndrome. Front. Endocrinol. 10, 189 (2019).
Singh, V., Kaur, R., Kumari, P., Pasricha, C. & Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta. 548, 117487 (2023).
Haydinger, C. D., Ashander, L. M., Tan, A. C. R. & Smith, J. R. Intercellular adhesion molecule 1: more than a leukocyte adhesion molecule. Biology 12, 743 (2023).
Rashad, N. M. et al. Intercellular adhesion molecule-1 expression and serum levels as markers of pre-clinical atherosclerosis in polycystic ovary syndrome. J. ovarian Res. 12, 1–12 (2019).
Jilani, T. N. & Siddiqui, A. H. in StatPearls [Internet] (StatPearls Publishing, 2023).
Rahman, F. A. & Krause, M. P. PAI-1, the plasminogen system, and skeletal muscle. Int. J. Mol. Sci. 21, 7066 (2020).
Bounds, E. J. & Kok, S. (2017). J. D dimer.
Lindholm, Å. et al. Tissue plasminogen activator and plasminogen activator inhibitor 1 in obese and lean patients with polycystic ovary syndrome. Gynecol. Endocrinol. 26, 743–748 (2010).
Moin, A. S. M. et al. Metabolic consequences of obesity on the hypercoagulable state of polycystic ovary syndrome. Sci. Rep. 11, 5320 (2021).
Atiomo, W. U. et al. The plasminogen activator system in women with polycystic ovary syndrome. Fertil. Steril. 69, 236–241 (1998).
Atiomo, W. U. et al. Raised plasminogen activator inhibitor-1 (PAI-1) is not an independent risk factor in the polycystic ovary syndrome (PCOS). Clinical endocrinology. 52, (2000).
Sprung, V. S. et al. Endothelial function measured using flow-mediated dilation in polycystic ovary syndrome: a meta‐analysis of the observational studies. Clin. Endocrinol. 78, 438–446 (2013).
Moreau, K. L. Modulatory influence of sex hormones on vascular aging. Am. J. Physiol. Heart Circ. Physiol. 316, H522–H526 (2019).
Wenner, M. M., Taylor, H. S. & Stachenfeld, N. S. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am. J. Physiology-Endocrinology Metabolism. 305, E818–E825 (2013).
Usselman, C. W. et al. Androgens drive microvascular endothelial dysfunction in women with polycystic ovary syndrome: role of the endothelin B receptor. J. Physiol. 597, 2853–2865 (2019).
Saxena, P., Prakash, A., Nigam, A. & Mishra, A. Polycystic ovary syndrome: Is obesity a sine qua non? A clinical, hormonal, and metabolic assessment in relation to body mass index. Indian J. Endocrinol. Metabol. 16, 996–999 (2012).
Ketel, I. J. G. et al. Insulin-induced capillary recruitment is impaired in both lean and obese women with PCOS. Hum. Reprod. 26, 3130–3137. https://doi.org/10.1093/humrep/der296 (2011).
Tarkun, I. et al. Endothelial dysfunction in young women with polycystic ovary syndrome: relationship with insulin resistance and low-grade chronic inflammation. J. Clin. Endocrinol. Metabolism. 89, 5592–5596 (2004).
Bird, S. T., Hartzema, A. G., Brophy, J. M., Etminan, M. & Delaney, J. A. Risk of venous thromboembolism in women with polycystic ovary syndrome: a population-based matched cohort analysis. Cmaj 185, E115–E120 (2013).
Juhan-Vague, I., Alessi, M. C., Mavri, A. & Morange, P. E. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J. Thromb. Haemost. 1, 1575–1579. https://doi.org/10.1046/j.1538-7836.2003.00279.x (2003).
Atkin, S. L., Butler, A. E., Jamialahmadi, T. & Sahebkar, A. PCSK7 levels in women with and without PCOS. J. Clin. Translational Endocrinol. 38, 100376 (2024).
Palomba, S. et al. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update. 21, 575–592 (2015).
Barry, J. A., Azizia, M. M. & Hardiman, P. J. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. Update. 20, 748–758 (2014).
Satyaraddi, A. et al. Body Composition, Metabolic Characteristics, and Insulin Resistance in Obese and Nonobese Women with Polycystic Ovary Syndrome. J. Hum. Reprod. Sci. 12, 78–84. https://doi.org/10.4103/jhrs.JHRS_2_19 (2019).
Polak, A. M. et al. Body Composition, Serum Concentrations of Androgens and Insulin Resistance in Different Polycystic Ovary Syndrome Phenotypes. J. Clin. Med. 9 https://doi.org/10.3390/jcm9030732 (2020).
Sathyapalan, T., Al-Qaissi, A., Kilpatrick, E. S., Dargham, S. R. & Atkin, S. L. Anti‐Müllerian hormone measurement for the diagnosis of polycystic ovary syndrome. Clin. Endocrinol. 88, 258–262 (2018).
Sathyapalan, T. et al. Salivary testosterone measurement in women with and without polycystic ovary syndrome. Sci. Rep. 7, 3589 (2017).
Cunningham, T. K. et al. Association of vitamin D metabolites with embryo development and fertilization in women with and without PCOS undergoing subfertility treatment. Front. Endocrinol. 10, 13 (2019).
Butler, A. E., Moin, A. S. M., Sathyapalan, T. & Atkin, S. L. Components of the complement cascade differ in polycystic ovary syndrome. Int. J. Mol. Sci. 23, 12232 (2022).
Kraemer, S. et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: a SOMAmer-based, streamlined multiplex proteomic assay. PloS one. 6, e26332 (2011).
Author information
Authors and Affiliations
Contributions
P.B., M.N., S.A.N., H.H., A. S.-N. T. and A.E.B. wrote the main manuscript and M.N. prepared Figure 1. T.S. supervised clinical studies and data collection. A.E.B. analyzed the data. S.L.A.: contributed to the conceptualization, study design, and data interpretation. All authors contributed to the reviewing and editing of the manuscript and approved the final version. Alexandra E Butler is the guarantor of this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Borde, P., Niinuma, S.A., Habib, H. et al. Dysregulation of Endothelial cell markers in polycystic ovary syndrome. Sci Rep (2026). https://doi.org/10.1038/s41598-026-40533-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-40533-3