Abstract
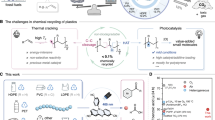
Chemical upcycling has the potential to turn plastic wastes into valuable chemicals and promote the circularity of plastics. Polysulfones and relevant composites are plastics with notable chemical durability, resistance and survivability. They are known as super engineering plastics, but they pose enormous challenges for reuse and upcycling. Here we develop a strategy for the chemical upcycling of polysulfones through copper-photocatalysed dual desulfonylative chlorination at ambient conditions, affording high-value-added dichlorinated diaryl ethers. The key to this strategy is cascade homolytic ipso-aromatic substitutions with high selectivity using the chlorine radical. Various types of polysulfones were successfully depolymerized and functionalized, including 12 examples of resins and eight examples of end-of-life plastics. Through gram-scale upcycling and mixed-plastic treatment, we further demonstrated the excellent compatibility and practicality of our strategy. Overall, this work establishes a viable approach for upcycling waste plastics into valuable chemicals, thus contributing to more sustainable plastic waste management and to realizing the circular economy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text or the supplementary materials.
References
Stubbins, A., Law, K. L., Muñoz, S. E., Bianchi, T. S. & Zhu, L. Plastics in the Earth system. Science 373, 51–55 (2021).
Stegmann, P., Daioglou, V., Londo, M., van Vuuren, D. P. & Junginger, M. Plastic futures and their CO2 emissions. Nature 612, 272–276 (2022).
Zuin, V. G. & Kümmerer, K. Chemistry and materials science for a sustainable circular polymeric economy. Nat. Rev. Mater. 7, 76–78 (2022).
Vidal, F. et al. Designing a circular carbon and plastics economy for a sustainable future. Nature 626, 45–57 (2024).
Korley, L. T. J., Epps, T. H., Helms, B. A. & Ryan, A. J. Toward polymer upcycling—adding value and tackling circularity. Science 373, 66–69 (2021).
Jehanno, C. et al. Critical advances and future opportunities in upcycling commodity polymers. Nature 603, 803–814 (2022).
Zhou, H. et al. Plastic waste valorization by leveraging multidisciplinary catalytic technologies. ACS Catal. 12, 9307–9324 (2022).
Chu, S. et al. Photocatalytic conversion of plastic waste: from photodegradation to photosynthesis. Adv. Energy Mater. 12, 2200435 (2022).
Ellis, L. D. et al. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 4, 539–556 (2021).
Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 370, 437–441 (2020).
Conk, R. J. et al. Catalytic deconstruction of waste polyethylene with ethylene to form propylene. Science 377, 1561–1566 (2022).
Conk, R. J. et al. Polyolefin waste to light olefins with ethylene and base-metal heterogeneous catalysts. Science 385, 1322–1327 (2024).
Huang, Z. et al. Chemical recycling of polystyrene to valuable chemicals via selective acid-catalyzed aerobic oxidation under visible light. J. Am. Chem. Soc. 144, 6532–6542 (2022).
Zhao, B. et al. Selective upcycling of polyolefins into high-value nitrogenated chemicals. J. Am. Chem. Soc. 146, 28605–28611 (2024).
DelRe, C. et al. Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature 592, 558–563 (2021).
Sun, B. et al. Valorization of waste biodegradable polyester for methyl methacrylate production. Nat. Sustain. 6, 712–719 (2023).
Ahrens, A. et al. Catalytic disconnection of C–O bonds in epoxy resins and composites. Nature 617, 730–737 (2023).
Clarke, R. W. et al. Manufacture and testing of biomass-derivable thermosets for wind blade recycling. Science 385, 854–860 (2024).
Fink, J. K. in High Performance Polymers (ed. Fink, J. K.) Ch. 7, 177–208 (William Andrew Publishing, 2014).
Mittal, V. in High Performance Polymers and Engineering Plastics (ed. Mittal, V.) Ch. 1, 1–20 (Wiley, 2011).
Wu, S. & Madkour, T. M. in Polymer Data Handbook 2nd edn (ed. Mark, J. E.) 617–620 (Oxford Univ. Press, 2009).
Maximize Market Research. Polysulfone market–industry analysis, by grade, applications, region and forecast (2023–2029). https://www.maximizemarketresearch.com/market-report/global-polysulfone-market/71241/ (Maximize Market Research, 2023).
Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies. 1st edn (CRC Press, 2007).
Benson, S. W. Thermochemistry and kinetics of sulfur-containing molecules and radicals. Chem. Rev. 78, 23–35 (1978).
Minami, Y., Inagaki, Y., Tsuyuki, T., Sato, K. & Nakajima, Y. Hydroxylation–depolymerization of oxyphenylene-based super engineering plastics to regenerate arenols. JACS Au 3, 2323–2332 (2023).
McGrail, P. T. Polyaromatics. Polym. Int. 41, 103–121 (1996).
Bart, J. C. J. Additives in Polymers: Industrial Analysis and Applications (John Wiley & Sons, 2005).
Zeng, D., Ma, Y., Deng, W.-P., Wang, M. & Jiang, X. Divergent sulfur(vi) fluoride exchange linkage of sulfonimidoyl fluorides and alkynes. Nat. Synth. 1, 455–463 (2022).
Meng, J., Zhou, Y., Li, D. & Jiang, X. Degradation of plastic wastes to commercial chemicals and monomers under visible light. Sci. Bull. 68, 1522–1530 (2023).
Ji, L., Meng, J., Li, C., Wang, M. & Jiang, X. From polyester plastics to diverse monomers via low-energy upcycling. Adv. Sci. 11, 2403002 (2024).
Wang, X. et al. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem. 10, 1180–1189 (2018).
Hillman, S. A. J. et al. Why do sulfone-containing polymer photocatalysts work so well for sacrificial hydrogen evolution from water? J. Am. Chem. Soc. 144, 19382–19395 (2022).
Tay, N. E. S. et al. 19F- and 18F-arene deoxyfluorination via organic photoredox-catalysed polarity-reversed nucleophilic aromatic substitution. Nat. Catal. 3, 734–742 (2020).
Zhou, Y., Hu, D., Li, D. & Jiang, X. Uranyl-photocatalyzed hydrolysis of diaryl ethers at ambient environment for the directional degradation of 4-O-5 lignin. JACS Au 1, 1141–1146 (2021).
Wang, S., Wang, H. & König, B. Photo-induced thiolate catalytic activation of inert Caryl-hetero bonds for radical borylation. Chem 7, 1653–1665 (2021).
Mahadevi, A. S. & Sastry, G. N. Cation–π interaction: its role and relevance in chemistry, biology, and material science. Chem. Rev. 113, 2100–2138 (2013).
Bowry, S. K., Gatti, E. & Vienken, J. Contribution of polysulfone membranes to the success of convective dialysis therapies. Contrib. Nephrol. 173, 110–118 (2011).
Ali, R. S. A. E., Meng, J. & Jiang, X. Multimode photo-CSTR (continuous stirred tank reactor) setup for heterogeneous photocatalytic processes. Org. Process Res. Dev. 28, 1683–1689 (2023).
Doyle, K. J., Tran, H., Baldoni-Olivencia, M., Karabulut, M. & Hoggard, P. E. Photocatalytic degradation of dichloromethane by chlorocuprate(ii) ions. Inorg. Chem. 47, 7029–7034 (2008).
Mereshchenko, A. S. et al. Photochemistry of copper(ii) chlorocomplexes in acetonitrile: trapping the ligand-to-metal charge transfer excited state relaxations pathways. Chem. Phys. Lett. 615, 105–110 (2014).
Treacy, S. M. & Rovis, T. Copper catalyzed C(sp3)–H bond alkylation via photoinduced ligand-to-metal charge transfer. J. Am. Chem. Soc. 143, 2729–2735 (2021).
Bowman, W. R. & Storey, J. M. D. Synthesis using aromatic homolytic substitution—recent advances. Chem. Soc. Rev. 36, 1803–1822 (2007).
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (grant numbers 22125103 and 21971065 to X.J. and 22301077 to Y.Z.), the Science and Technology Commission of Shanghai Municipality (grant number 22JC140100 to X.J.) and the Shanghai Pujiang Program (grant number 22PJ1403200 to Y.Z.). R.H. acknowledges support from the PhD Scientific Research and Innovation Foundation of the Education Department of Hainan Province Joint Project of Sanya Yazhou Bay Science and Technology City (grant number HSPHDSRF-2024-14-003).
Author information
Authors and Affiliations
Contributions
Conceptualization: R.H., Y.Z. and X.J. Methodology: R.H., Y.Z. and X.J. Formal analysis: C.L. and Y.Z. Project administration: Y.Z. and X.J. Supervision: Y.Z., D.L. and X.J. Writing—original draft: R.H., Y.Z. and X.J. Writing—review and editing: R.H., Y.Z. and X.J.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Meng Wang, Fan Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Text, Figs. 1–170 and Tables 1–6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, R., Zhao, Y., Li, C. et al. Photocatalytic upcycling of polysulfones at ambient conditions. Nat Sustain 8, 818–826 (2025). https://doi.org/10.1038/s41893-025-01569-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41893-025-01569-x