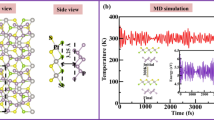
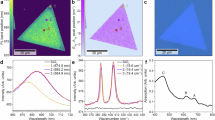
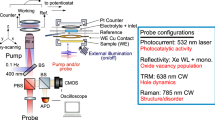
Abstract
Achieving efficient carrier separation in transition-metal-oxide semiconductors is crucial for their applications in optoelectronic and catalytic devices. However, the substantial disparity in mobility between holes and electrons heavily limits device performance. Here we develop a general strategy for enhancing hole mobility via reducing their effective mass through metal vacancy (VM) management. The introduction of VM yields remarkable improvements in hole mobility: 430% for WO3, 350% for TiO2 and 270% for Bi2O3. To illustrate the importance of this finding, we applied the VM concept to photoelectrochemical water splitting, where efficient carrier separation is highly coveted. In particular, VM-WO3 achieves a 4.4-fold enhancement in photo-to-current efficiency, yielding a performance of 4.8 mA cm−2 for both small- and large-scale photoelectrodes with exceptional stability for over 120 h.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Xiao, Y. et al. Band structure engineering and defect control of Ta3N5 for efficient photoelectrochemical water oxidation. Nat. Catal. 3, 932–940 (2020).
Nishiyama, H. et al. Photocatalytic solar hydrogen production from water on a 100 m2 scale. Nature 598, 304–307 (2021).
Cho, H.-H. et al. A semiconducting polymer bulk heterojunction photoanode for solar water oxidation. Nat. Catal. 4, 431–438 (2021).
Tan, J. et al. Hydrogel protection strategy to stabilize water-splitting photoelectrodes. Nat. Energy 7, 537–547 (2022).
Zeng, G. et al. Development of a photoelectrochemically self-improving Si/GaN photocathode for efficient and durable H2 production. Nat. Mater. 20, 1130–1135 (2021).
Ager, J. W., Shaner, M. R., Walczak, K. A., Sharp, I. D. & Ardo, S. Experimental demonstrations of spontaneous, solar-driven photoelectrochemical water splitting. Energy Environ. Sci. 8, 2811–2824 (2015).
Roger, I., Shipman, M. A. & Symes, M. D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 1, 0003 (2017).
Luo, Y., Zhang, Z., Chhowalla, M. & Liu, B. Recent advances in design of electrocatalysts for high-current-density water splitting. Adv. Mater. 34, 2108133 (2022).
Liao, W. et al. Boosting nitrogen activation via Ag nanoneedle arrays for efficient ammonia synthesis. ACS Nano 17, 411–420 (2023).
Yang, W., Prabhakar, R. R., Tan, J., Tilley, S. D. & Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 48, 4979–5015 (2019).
Wang, S., Liu, G. & Wang, L. Crystal facet engineering of photoelectrodes for photoelectrochemical water splitting. Chem. Rev. 119, 5192–5247 (2019).
Sheng, X., Xu, T. & Feng, X. Rational design of photoelectrodes with rapid charge transport for photoelectrochemical applications. Adv. Mater. 31, 1805132 (2019).
Ding, C., Shi, J., Wang, Z. & Li, C. Photoelectrocatalytic water splitting: significance of cocatalysts, electrolyte, and interfaces. ACS Catal. 7, 675–688 (2016).
He, B. et al. General and robust photothermal-heating-enabled high-efficiency photoelectrochemical water splitting. Adv. Mater. 33, 2004406 (2021).
Wang, J. et al. Subsurface engineering induced fermi level de-pinning in metal oxide semiconductors for photoelectrochemical water splitting. Angew. Chem. Int. Ed. 62, e202217026 (2023).
Andrei, V. et al. Long-term solar water and CO2 splitting with photoelectrochemical BiOI–BiVO4 tandems. Nat. Mater. 21, 864–868 (2022).
Jun, S. E. et al. Atomically dispersed iridium catalysts on silicon photoanode for efficient photoelectrochemical water splitting. Nat. Commun. 14, 609 (2023).
Gao, R. T. et al. Single-atomic-site platinum steers photogenerated charge carrier lifetime of hematite nanoflakes for photoelectrochemical water splitting. Nat. Commun. 14, 2640 (2023).
Zhai, L. et al. Epitaxial growth of highly symmetrical branched noble metal-semiconductor heterostructures with efficient plasmon-induced hot-electron transfer. Nat. Commun. 14, 2538 (2023).
Fu, J. et al. Interface engineering of Ta3N5 thin film photoanode for highly efficient photoelectrochemical water splitting. Nat. Commun. 13, 729 (2022).
Guo, Y. et al. Efficient interfacial electron transfer induced by hollow-structured ZnIn2S4 for extending hot electron lifetimes. Energy Environ. Sci. 16, 3462–3473 (2023).
Pei, H. et al. A novel BiVO4/P3HT photoanode for highly efficient water oxidation and its hole energy offset effect with CuPc derivative. Appl. Catal. B 318, 121865 (2022).
Ahn, H.-J. et al. Utilizing a siloxane-modified organic semiconductor for photoelectrochemical water splitting. ACS Energy Lett. 8, 2595–2602 (2023).
Lin, R. et al. Quantitative study of charge carrier dynamics in well-defined WO3 nanowires and nanosheets: Insight into the crystal facet effect in photocatalysis. J. Am. Chem. Soc. 140, 9078–9082 (2018).
Liu, G. et al. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 132, 11642–11648 (2010).
Guan, M. et al. Vacancy associates promoting solar-driven photocatalytic activity of ultrathin bismuth oxychloride nanosheets. J. Am. Chem. Soc. 135, 10411–10417 (2013).
Wang, J. et al. Enhanced photoelectrochemical degradation of tetracycline hydrochloride with FeOOH and Au nanoparticles decorated WO3. Chem. Eng. J. 407, 127195 (2021).
Du, J., Li, C., Wang, X., Shi, X. & Liang, H.-P. Electrochemical synthesis of cation vacancy-enriched ultrathin bimetallic oxyhydroxide nanoplatelets for enhanced water oxidation. ACS Appl. Mater. Interfaces 11, 25958–25966 (2019).
Li, X., Wang, X., Ma, L. & Huang, W. Solvation structures in aqueous metal-ion batteries. Adv. Energy Mater. 12, 2202068 (2022).
Melzer, D. et al. Design and synthesis of highly active MoVTeNb-oxides for ethane oxidative dehydrogenation. Nat. Commun. 10, 4012 (2019).
Ye, C. et al. Activating metal oxides nanocatalysts for electrocatalytic water oxidation by quenching-induced near-surface metal atom functionality. J. Am. Chem. Soc. 143, 14169–14177 (2021).
Wang, S. et al. New BiVO4 dual photoanodes with enriched oxygen vacancies for efficient solar-driven water splitting. Adv. Mater. 30, 1800486 (2018).
Lu, Y. et al. Boosting charge transport in BiVO4 photoanode for solar water oxidation. Adv. Mater. 34, 2108178 (2022).
Zhang, D. et al. Regulating spin polarization through cationic vacancy defects in Bi4Ti3O12 for enhanced molecular oxygen activation. Angew. Chem. Int. Ed. 62, e202303807 (2023).
Wang, D. et al. Neighboring cationic vacancy assisted adsorption optimization on single-atom sites for improved oxygen evolution. ACS Catal. 12, 12458–12468 (2022).
Zhai, P. et al. Engineering single-atomic ruthenium catalytic sites on defective nickel-iron layered double hydroxide for overall water splitting. Nat. Commun. 12, 4587 (2021).
Promchana, P., Choojun, K., Limphirat, W., Poo-arporn, Y. & Sooknoi, T. Selective acetylene removal from ethylene-rich feed by cross-metathesis over supported WO3 catalysts. Appl. Catal. A 650, 118972 (2023).
Hou, T. et al. Operando oxygen vacancies for enhanced activity and stability toward nitrogen photofixation. Adv. Energy Mater. 9, 1902319 (2019).
Chen, W. et al. Single tungsten atoms supported on MOF-derived n-doped carbon for robust electrochemical hydrogen evolution. Adv. Mater. 30, e1800396 (2018).
Macdonald, T. J. et al. Phosphorene nanoribbon-augmented optoelectronics for enhanced hole extraction. J. Am. Chem. Soc. 143, 21549–21559 (2021).
Pan, L. et al. Cu2O photocathodes with band-tail states assisted hole transport for standalone solar water splitting. Nat. Commun. 11, 318 (2020).
Li, W. et al. Light-activated interlayer contraction in two-dimensional perovskites for high-efficiency solar cells. Nat. Nanotechnol. 17, 45–52 (2022).
Lang, F. et al. Efficient minority carrier detrapping mediating the radiation hardness of triple-cation perovskite solar cells under proton irradiation. Energy Environ. Sci. 12, 1634–1647 (2019).
Hages, C. J. et al. Identifying the real minority carrier lifetime in nonideal semiconductors: a case study of kesterite materials. Adv. Energy Mater. 7, 1700167 (2017).
de Araújo, M. A. et al. Contrasting transient photocurrent characteristics for thin films of vacuum-doped “grey” TiO2 and “grey” Nb2O5. Appl. Catal. B 237, 339–352 (2018).
Gupta, T., Samriti, Cho, J. & Prakash, J. Hydrothermal synthesis of TiO2 nanorods: Formation chemistry, growth mechanism, and tailoring of surface properties for photocatalytic activities. Mater. Today Chem. 20, 100428 (2021).
Yu, X. et al. Facile synthesis of α/β-Bi2O3 hetero-phase junction by a solvothermal method for enhanced photocatalytic activities. Mol. Catal. 503, 111431 (2021).
Wang, F., Di Valentin, C. & Pacchioni, G. Electronic and structural properties of WO3: a systematic hybrid DFT study. J. Phys. Chem. C 115, 8345–8353 (2011).
Tosoni, S., Di Liberto, G. & Pacchioni, G. Structures and properties of Pd nanoparticles intercalated in layered TiO2: a computational study. Catal. Today 382, 96–103 (2021).
Ugolotti, A., Dolce, M. & Di Valentin, C. Vitamin C affinity to TiO2 nanotubes: a computational study by hybrid density functional theory calculations. Nanomaterials 14, 261 (2024).
Meng, Q. et al. Efficient BiVO4 photoanodes by postsynthetic treatment: remarkable improvements in photoelectrochemical performance from facile borate modification. Angew. Chem. Int. Ed. 58, 19027–19033 (2019).
Ran, L. et al. Conformal macroporous inverse opal oxynitride-based photoanode for robust photoelectrochemical water splitting. J. Am. Chem. Soc. 143, 7402–7413 (2021).
Yi, S.-S., Wulan, B.-R., Yan, J.-M. & Jiang, Q. Highly efficient photoelectrochemical water splitting: surface modification of cobalt-phosphate-loaded Co3O4/Fe2O3 p–n heterojunction nanorod arrays. Adv. Funct. Mater. 29, 1801902 (2019).
Zhang, H. et al. Gradient tantalum-doped hematite homojunction photoanode improves both photocurrents and turn-on voltage for solar water splitting. Nat. Commun. 11, 4622 (2020).
Huang, M. et al. Twin structure in BiVO4 photoanodes boosting water oxidation performance through enhanced charge separation and transport. Adv. Energy Mater. 8, 1802198 (2018).
Acknowledgements
We thank the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (grant no. 52121004), National Natural Science Foundation of China (grant nos. 22376222, 52372253, 22002189 and 52202125), the Science and Technology lnnovation Program of Hunan Province (grant no. 2023RC1012), Central South University Research Programme of Advanced Interdisciplinary Studies (grant no. 2023QYJC012), Central South University Innovation-Driven Research Programme (grant no. 2023CXQD042) and China Postdoctoral Science Foundation (grant nos. 2022M723547, 2022M713548 and 2023T160735). We also acknowledge the funding and support from the Deutsche Forschungsgemeinschaft (DFG) under Germany’s Excellence Strategy EXC 2089/1-390776260, the Bavarian Program Solar Technologies Go Hybrid (SolTech), the Center for NanoScience (CeNS) and the European Commission through the ERC grants CATALIGHT and SURFLIGHT. Y.K. and E.C. acknowledge the DFG excellence research cluster e-conversion for financing via the students exchange program the 4-month reseach stay of Y.K. from LMU to CSU. We are grateful for resources from the High Performance Computing Center of Central South University. We acknowledge the help from Beam Lines BL01C1 in the National Synchrotron Radiation Research Center (Hsinchu, Taiwan) for various synchrotron-based measurements.
Author information
Authors and Affiliations
Contributions
M.L. and E.C. supervised the whole project. J.W. and W.L. conceived the research and designed the experiments. K.L. carried out the DFT calculations and wrote the corresponding section. J.W., W.L., Y.K., H.X., Y.C., Q.W., T.L. and J.C. synthesized the samples, performed the electrochemical experiments and catalyst characterizations, and analysed the results. H.L., S.C., E.P., F.L., L.J., C.L., L.C. and J.F. contributed to the manuscript editing and revising. T.-S.C. conducted the XAS measurements. J.W. and H.X. performed the SCLC measurements. All authors read and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–56, Discussion and Tables 1–8.
Supplementary Data 1
Atomic coordinates of DFT calculation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Liu, K., Liao, W. et al. Metal vacancies in semiconductor oxides enhance hole mobility for efficient photoelectrochemical water splitting. Nat Catal 8, 229–238 (2025). https://doi.org/10.1038/s41929-025-01300-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01300-1
This article is cited by
-
Near-unity CO2-to-ethylene photoconversion over low coordination single-atom catalysts
Nature Communications (2026)
-
Determining kinetics of H2O2 evolution from photoelectrochemical water oxidation
Nature Communications (2025)
-
Lighten the holes
Nature Catalysis (2025)
-
Oxygen vacancy and Schottky junction synergistically regulated Ag@SnO2 interface carrier transport performance for triethylamine gas sensing monitoring
Microchimica Acta (2025)