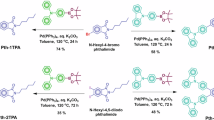
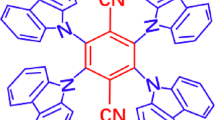
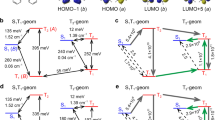
Abstract
Advanced photonic materials showing two-photon absorption (2PA) have been widely explored to develop three-dimensional imaging, micro and nanofabrication, all-optical switching, lithography on a nanoscale and many other enabling technologies. These all require nonlinear absorption chromophores with intrinsic 2PA cross-sections and long-term photo- and thermal stability. Here, we disclose the very first example of the dipolar carbene-metal-amide (CMA) material showing a enhanced 2PA cross-section up to 105 GM. Overall molecular design considerations such as extended π-conjugation (to increase polarizability), minimizing the singlet-triplet energy gap (ΔEST), and using heavy metal atoms are the first design principles to obtain bright one- and two-photon excited thermally activated delayed fluorescence (TADF) material, showing one of the highest radiative rate of 2.18·106 s-1 across CMA materials. Bright red CMA 2P-TADF material shows excellent photostability (LT50 = 3 h) to 20 mW femtosecond pulsed laser excitation at 1000 nm, encouraging further CMA exploration for future applications in advanced photonic technologies requiring third-order nonlinear optical properties.

Similar content being viewed by others
Data availability
The data that support the plots within this paper and the Supplementary Information and other findings of this study are available from the corresponding authors upon reasonable request.
References
Walker, E. & Rentzepis, P. M. A new dimension. Nat. Photonics 2, 406–408 (2008).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005).
Lesani, P. et al. Design principles and biological applications of red-emissive two-photon carbon dots. Commun. Mater. 2, 108 (2021).
Collins, H. A. et al. Blood-vessel closure using photosensitizers engineered for two-photon excitation. Nat. Photonics 2, 420–424 (2008).
Liang, Z. X. et al. Two-photon absorption under few-photon irradiation for optical nanoprinting. Nat. Comm. 16, 2086 (2025).
Gieseking, R. L., Mukhopadhyay, S., Risko, C., Marder, S. R. & Brédas, J.-L. 25th Anniversary Article: Design of polymethine dyes for all-optical switching applications: guidance from theoretical and computational studies. Adv. Mater. 26, 68–84 (2014).
Pascal, S., David, S., Andraud, C. & Maury, O. Near-infrared dyes for two-photon absorption in the short-wavelength infrared: strategies towards optical power limiting. Chem. Soc. Rev. 50, 6613–6658 (2021).
Mageswari, G. V., Chitose, Y., Tsuchiya, Y., Lin, H.-H. & Adachi, C. Rational molecular design for balanced locally excited and charge- transfer nature for two-photon absorption phenomenon and highly efficient TADF-based OLEDs. Angew. Chem. Int. Ed. 64, e202420417 (2025).
Katan et al. Effects of (multi)branching of dipolar chromophores on photophysical properties and two-photon absorption. J. Phys. Chem. A 109, 3024 (2005).
Collings, J. C. et al. The synthesis and one- and two-photon optical properties of dipolar, quadrupolar and octupolar donor–acceptor molecules containing dimesitylboryl groups. Chem. Eur. J. 15, 198–208 (2009).
Albota, M. et al. Design of organic molecules with large two-photon absorption cross sections. Science 281, 1653–1656 (1998).
Zhang, L. & Humphrey, M. G. Multiphoton absorption at metal alkynyl complexes. Coord. Chem. Rev. 473, 214820 (2022).
Brannan, A. C. et al. Unity Fluorescent carbene-gold(I)-acetylide complexes with two-photon absorption and energy-efficient blue FOLED. J. Mater. Chem. C. 12, 13545–13554 (2024).
Jensen, L., Mende, M., Schrameyer, S., Jupé, M. & Ristau, D. Role of two-photon absorption in Ta2O5 thin films in nanosecond laser-induced damage. Opt. Lett. 37, 4329–4329 (2012).
Price, R. S. et al. Polymer monoliths containing two-photon absorbing phenylenevinylene platinum(II) acetylide chromophores for optical power limiting. ACS Appl. Mater. Interfaces 7, 10795–10805 (2015).
Cheriton, R. et al. Two-photon photocurrent in InGaN/GaN nanowire intermediate band solar cells. Commun. Mater. 1, 1–7 (2020).
Terenziani, F., Katan, C., Badaeva, E., Tretiak, S. & Blanchard-Desce, M. Enhanced two-photon absorption of organic chromophores: theoretical and experimental assessments. Adv. Mater. 20, 4641–4678 (2008).
Elgadi, S. A., Mayder, D. M., Hojo, R. & Hudson, Z. M. Thermally activated delayed fluorescence and room- temperature phosphorescence in sulfidoazatriangulene-based materials and their S-oxides. Adv. Opt. Mater. 11, 2202754 (2023).
Chitose, Y. et al. Unlocking dual functionality in triazine-based emitters: synergistic enhancement of two-photon absorption and TADF-OLED performance with electron-withdrawing substituents. Adv. Mater. 37, 2509857 (2025).
Di, D. et al. High-performance light-emitting diodes based on carbene-metal-amides. Science 356, 159–163 (2017).
Brannan, A. C. et al. Deep-blue and fast delayed fluorescence from carbene-metal-amides for highly efficient and stable organic light-emitting diodes. Adv. Mater. 36, 2404357 (2024).
Li, J. et al. Two-coordinate copper(I)/NHC complexes: dual emission properties and ultralong room-temperature phosphorescence. Angew. Chem. Int. Ed. Engl. 59, 8210–8217 (2020).
Chotard, F., Sivchik, V., Linnolahti, M., Bochmann, M. & Romanov, A. S. Mono- versus bicyclic carbene metal amide photoemitters: which design leads to the best performance?. Chem. Mater. 32, 6114–6122 (2020).
Yang, J.-G. et al. Highly efficient thermally activated delayed fluorescence from pyrazine-fused carbene Au(I) emitters. Chem. Eur. J. 27, 17834–17842 (2021).
Riley, C., Nguyen Le Phuoc, M., Linnolahti, M. & Romanov, A. S. Linear gold(I) halide complexes with a diamidocarbene ligand: synthesis, reactivity, and phosphorescence. Organometallics 43, 1687–1697 (2023).
Turro, N. J., Ramamurthy, V. & Scaiano, J. C. Modern Molecular Photochemistry of Organic Molecules (University Science Books, 2010).
Panchompoo, J., Aldous, L., Baker, M., Wallace, M. I. & Compton, R. G. One-step synthesis of fluorescein modified nano-carbon for Pd(II) detection via fluorescence quenching. Analyst 137, 2054 (2012).
Minò, A., Cinelli, G., Lopez, F. & Ambrosone, L. Optical behavior of Nile Red in organic and aqueous media environments. Appl. Sci. 13, 638–638 (2023).
de Reguardati, S., Pahapill, J., Mikhailov, A., Stepanenko, Y. & Rebane, A. High-accuracy reference standards for two-photon absorption in the 680–1050 nm wavelength range. Opt. Express 24, 9053 (2016).
Valandro, S. R., Jagadesan, P., Feng, F. & Schanze, K. Aggregation-enhanced two-photon absorption of anionic conjugated polyelectrolytes. J. Phys. Chem. Lett. 11, 8292–8296 (2020).
Tsuchiya, Y. et al. Exact solution of kinetic analysis for thermally activated delayed fluorescence materials. J. Phys. Chem. A 125, 8074–8089 (2021).
Tang, R. et al. Au(I)-TADF emitters for high efficiency full-color vacuum-deposited OLEDs and TADF-sensitized fluorescent oleds with ultrahigh brightness and prolonged operational lifetime. Adv. Opt. Mater. 11, 2300950 (2023).
Valverde, D. et al. Computational investigations of the detailed mechanism of reverse intersystem crossing in inverted singlet–triplet gap molecules. ACS Appl. Mater. Interfaces 16, 66991–67001 (2024).
Castet, F., Tonnelé, C., Muccioli, L. & Champagne, B. Predicting the second-order nonlinear optical responses of organic materials: the role of dynamics. Acc. Chem. Res. 55, 3716–3726 (2022).
Ramos, T. N., Franco, L. R., Silva, D. L. & Canuto, S. Calculation of the one- and two-photon absorption spectra of water-soluble stilbene derivatives using a multiscale QM/MM approach. J. Chem. Phys. 159, 024309 (2023).
Reponen, A.-P. M. et al. Understanding spin-triplet excited states in carbene-metal-amides. Angew. Chem. Int. Ed. Engl. 63, e202402052 (2024).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Frisch, M. J. et al. Gaussian 16 Rev. A.03 (Gaussian Inc., 2016).
Etienne, T., Assfeld, X. & Monari, A. Toward a quantitative assessment of electronic transitions’ charge-transfer character. J. Chem. Theory Comput. 10, 3896–3905 (2014).
Dalton, a molecular electronic structure program, Release Dalton2020.1, see http://daltonprogram.org (2022).
Ramos, T. N., Silva, D. L., Cabral, B. J. C. & Canuto, S. On the spectral line width broadening for simulation of the two-photon absorption cross-section of para-nitroaniline in liquid environment. J. Mol. Liq. 301, 112405 (2020).
Wielgus, M. et al. Two-photon solvatochromism II: experimental and theoretical study of solvent effects on the two-photon absorption spectrum of Reichardt’s dye. Chem. Phys. Chem. 14, 3731–3739 (2013).
Uudsemaa, M., Trummal, A., de Reguardati, S., Callis, P. R. & Rebane, A. TD-DFT calculations of one- and two-photon absorption in Coumarin C153 and Prodan: attuning theory to experiment. Phys. Chem. Chem. Phys. 19, 28824–28833 (2017).
Beerepoot, M. T. P., Friese, D. H., List, N. H., Kongsted, J. & Ruud, K. Benchmarking two-photon absorption cross sections: performance of CC2 and CAM-B3LYP. Phys. Chem. Chem. Phys. 17, 19306–19314 (2015).
Paterson, M. J. et al. Benchmarking two-photon absorption with CC3 quadratic response theory, and comparison with density-functional response theory. J. Chem. Phys. 124, 054322 (2006).
Naim, C., Zaleśny, R. & Jacquemin, D. Two-photon absorption strengths of small molecules: reference cc3 values and benchmarks. J. Chem. Theory Comput. 20, 9093–9106 (2024).
Sałek, P. et al. Calculations of two-photon absorption cross sections by means of density-functional theory. Chem. Phys. Lett. 374, 446–452 (2003).
Thellamurege, N. M. & Li, H. Note: FixSol solvation model and FIXPVA2 tessellation scheme. J. Chem. Phys. 137, 246101 (2012).
Olsen, J. M. H. et al. The polarizable embedding library. J. Comput. Chem. 41, 2426–2433 (2020).
Sissa, C. et al. The effectiveness of essential-state models in the description of optical properties of branched push–pull chromophores. Phys. Chem. Chem. Phys. 12, 11715–11727 (2010).
Makarov, N. S., Drobizhev, M. & Rebane, A. Two-photon absorption standards in the 550–1600 nm excitation wavelength range. Opt. Express 16, 4029–4047 (2008).
Acknowledgements
A.S.R. acknowledges support from the Royal Society (grant nos. URF\R1\180288, RGF\EA\181008, URF\R\231014), EPSRC (grant code EP/K039547/1 and APP46952). M.L. acknowledges the Academy of Finland Flagship Programme, Photonics Research and Innovation (PREIN), decision 320166, the Finnish Grid and Cloud Infrastructure resources (urn:nbn:fi:research-infras-2016072533). N.L.P. acknowledges the Doctoral Programme in Science, Forestry and Technology (Lumeto, University of Eastern Finland). T.N.R. is a postdoctoral researcher of the Fonds de la Recherche Scientifique – FNRS” (F.R.S.–FNRS). We thank Dr Louise Natrajan, EPSRC and University of Manchester for access the Centre for Radiochemistry Research National Nuclear User’s Facility (NNUF, EP/T011289/1) to use FLS-1000 fluorometer. D.T.W.T. acknowledges Diamond Light Source for access to the DL-SAXS equipment (experiment number SM40538-1) supported by an EPSRC grant (EP/R042683/1), and instrument scientist Dr Paul Wady for their help and support during beamtime. L.M. thanks the Winton Programme and Harding Distinguished Postgraduate Scholarship for funding. A.J.G. thanks the Leverhulme Trust for an Early Career Fellowship (ECF-2022-445), the Knut and Alice Wallenberg Foundation for a Wallenberg Academy Fellows award (KAW 2023.0082), and the Swedish Research Council (VR) for a Starting Grant (2024-03915). European Union’s Horizon 2020 research and innovation programme grant agreement no. 101020167 (L.M. and A.J.G.).
Author information
Authors and Affiliations
Contributions
I.D.N. carried out synthesis and characterization, electrochemistry, UV-vis and photoluminescence spectroscopy and analysis, L.M. and A.J.G. performed transient absorption measurements and analysis, D.T.W.T. performed GIWAXS measurements and analysis, T.N.R., N.L.P., G.L., M.L., Y.O. performed theoretical calculations for the ground and excited states and calculations involving a two-photon process, C.T.S. assisted with initial collection of the two-photon absorption data and performed two-photon excited TADF lifetime measurements, G.F.S.W. performed single crystal experiment and refinement, M.B.-D., J.D. and I.D.N. performed two-photon absorption and luminescence experiments in fluid and solid state, A.S.R. conceived and designed the idea. J.D., M.L., Y.O. and A.S.R. planned the project and designed the experiments. I.D.N., Y.O., and A.S.R. wrote the manuscript. All authors contributed to the discussion of the results, analysis of the data, and reviewed and corrected the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nwosu, I.D., Matasović, L., Ramos, T.N. et al. Enhanced third-order optical nonlinearity in a dipolar carbene-metal-amide material with two-photon excited delayed fluorescence. Commun Chem (2026). https://doi.org/10.1038/s42004-026-01928-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-026-01928-5