Abstract
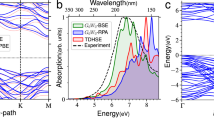
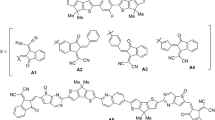
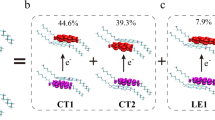
Identifying solid substrates that can effectively support donor-acceptor (DA) organic molecules without hindering their intrinsic charge transfer induced by ultraviolet and visible light is crucial for device fabrication and the precise control of photoinduced electric currents. Here, we demonstrate that graphene/SiC(000\(\overline{1}\)) interfaces preserve the optoelectronic response of three DA molecules: 1-amino-4-nitrobenzene, 1-amino-6-nitro-pyrene, and 1-fluoro-6-methoxy-pyrene. Although adsorption induces a substantial renormalization of the molecular quasiparticle gaps, the corresponding charge-transfer excitation energies exhibit relatively small redshift compared to the gas-phase molecules. This is attributed to the substrate-induced screening that reduces both the quasiparticle gap and the electron-hole binding energy, leading to a partial cancellation in the excitation energy. Additionally, we show that the intramolecular charge-transfer excitons retain their character upon adsorption, suggesting graphene/SiC(000\(\overline{1}\)) as a suitable platform for stable physisorption of DA chromophores and for enabling time-resolved studies of early-stage charge migration, opening pathways toward molecular optoelectronic architectures with minimal substrate interference.
Similar content being viewed by others
Data availability
All data generated and analyzed throughout this work are available from the corresponding authors upon reasonable request.
References
Thomsen, C. L., Thøgersen, J. & Keiding, S. R. Ultrafast charge-transfer dynamics: studies of p-nitroaniline in water and dioxane. J. Phys. Chem. A 102, 1062–1067 (1998).
Máximo-Canadas, M. & Borges Jr, I. Absorption spectra of p–nitroaniline derivatives: charge transfer effects and the role of substituents. J. Mol. Model. 30, 120 (2024).
Wang, S. et al. Rational design of hybridized local and charge transfer emitters towards high-performance fluorescent blue oleds. J. Mater. Chem. C. 11, 8196–8203 (2023).
Bergkamp, J. J., Decurtins, S. & Liu, S.-X. Current advances in fused tetrathiafulvalene donor–acceptor systems. Chem. Soc. Rev. 44, 863–874 (2015).
Amacher, A. et al. A quinoxaline-fused tetrathiafulvalene-based sensitizer for efficient dye-sensitized solar cells. Chem. Commun. 50, 6540–6542 (2014).
Mubarik, A., Shafiq, F., Wang, H.-R., Jiang, J. & Ju, X.-H. Theoretical design and evaluation of efficient small donor molecules for organic solar cells. J. Mol. Model. 29, 373 (2023).
Haseena, S. & Ravva, M. K. Theoretical studies on donor–acceptor based macrocycles for organic solar cell applications. Sci. Rep. 12, 15043 (2022).
Irfan, A. & Mahmood, A. Designing of efficient acceptors for organic solar cells: molecular modelling at dft level. J. Clust. Sci. 29, 359–365 (2018).
Meier, T. et al. Donor-acceptor properties of a single-molecule altered by on-surface complex formation. ACS Nano 11, 8413–8420 (2017).
Gilbert Gatty, M. et al. Hopping versus tunneling mechanism for long-range electron transfer in porphyrin oligomer bridged donor-acceptor systems. J. Phys. Chem. B 119, 7598–7611 (2015).
Metzger, R. M. Unimolecular electrical rectifiers. Chem. Rev. 103, 3803–3834 (2003).
He, Y., Li, N. & Brabec, C. J. Single-component organic solar cells with competitive performance. Org. Mater. 3, 228–244 (2021).
Duan, L., Qiao, J., Sun, Y. & Qiu, Y. Strategies to design bipolar small molecules for oleds: Donor-acceptor structure and non-donor-acceptor structure. Adv. Mater. 23, 1137–1144 (2011).
Kim, S.-Y., Kim, M.-J., Ahn, M., Lee, K.-M. & Wee, K.-R. Systematic energy band gap control of pyrene based donor-acceptor-donor molecules for efficient chemosensor. Dyes Pigments 191, 109362 (2021).
Calegari, F. et al. Ultrafast electron dynamics in phenylalanine initiated by attosecond pulses. Science 346, 336–339 (2014).
Lara-Astiaso, M. et al. Attosecond pump–probe spectroscopy of charge dynamics in tryptophan. J. Phys. Chem. Lett. 9, 4570–4577 (2018).
Calegari, F. & Martin, F. Open questions in attochemistry. Commun. Chem. 6, 184 (2023).
Vismarra, F. et al. Few-femtosecond electron transfer dynamics in photoionized donor–π–acceptor molecules. Nat. Chem. 16, 2017–2024 (2024).
Loriot, V. et al. Attosecond metrology of the two-dimensional charge distribution in molecules. Nat. Phys. 20, 765–769 (2024).
Mocci, D. et al. Ultrafast structural reorganization and charge dynamics in a photoionized donor-acceptor biphenyl molecule. Ultrafast Sci. 5, 0108 (2025).
Galli, M. et al. Generation of deep ultraviolet sub-2-fs pulses. Opt. Lett. 44, 1308–1311 (2019).
Reduzzi, M. et al. Direct temporal characterization of sub-3-fs deep uv pulses generated by resonant dispersive wave emission. Opt. Express 31, 26854–26864 (2023).
Colaizzi, L. et al. Few-femtosecond time-resolved study of the uv-induced dissociative dynamics of iodomethane. Nat. Commun. 15, 9196 (2024).
Wanie, V. et al. Capturing electron-driven chiral dynamics in UV-excited molecules. Nature 630, 109–115 (2024).
Hass, J. et al. Structural properties of the multilayer graphene/\(4h{{\rm{}}}-{{\rm{}}}{{\rm{Si}}}{{\rm{C}}}(000\overline{1})\) system as determined by surface x-ray diffraction. Phys. Rev. B 75, 214109 (2007).
Forbeaux, I., Themlin, J.-M. & Debever, J.-M. High-temperature graphitization of the 6H-SiC (0001) face. Surf. Sci. 442, 9–18 (1999).
Hass, J. et al. Highly ordered graphene for two dimensional electronics. Appl. Phys. Lett. 89, 143106 (2006).
Cavallucci, T. & Tozzini, V. Multistable rippling of graphene on sic: a density functional theory study. J. Phys. Chem. C. 120, 7670–7677 (2016).
Mansouri, M., Martín, F. & Díaz, C. Tunable doping and optoelectronic modulation in graphene-covered 4H-SiC surfaces. J. Phys. Chem. C. 129, 4155–4164 (2025).
Mansouri, M., Díaz, C. & Martín, F. Optoelectronic properties of electron-acceptor molecules adsorbed on Graphene/Silicon Carbide interfaces. Commun. Mater. 5, 117 (2024).
Bolotin, K. et al. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 146, 351–355 (2008).
Neaton, J. B., Hybertsen, M. S. & Louie, S. G. Renormalization of molecular electronic levels at metal-molecule interfaces. Phys. Rev. Lett. 97, 216405 (2006).
Garcia-Lastra, J. M., Rostgaard, C., Rubio, A. & Thygesen, K. S. Polarization-induced renormalization of molecular levels at metallic and semiconducting surfaces. Phys. Rev. B 80, 245427 (2009).
Garcia-Lastra, J. M. & Thygesen, K. S. Renormalization of optical excitations in molecules near a metal surface. Phys. Rev. Lett. 106, 187402 (2011).
Despoja, V., García de Abajo, F. J., Šunjić, M. & Novko, D. Quasiparticle spectra and excitons of organic molecules deposited on graphene and metallic substrates. Phys. Rev. B 88, 235437 (2013).
Deilmann, T. & Thygesen, K. S. Important role of screening the electron-hole exchange interaction for the optical properties of molecules near metal surfaces. Phys. Rev. B 99, 045133 (2019).
Aumiler, D., Wang, S., Chen, X. & Xia, A. Excited state localization and delocalization of internal charge transfer in branched push- pull chromophores studied by single-molecule spectroscopy. J. Am. Chem. Soc. 131, 5742–5743 (2009).
Sun, G. et al. Effect of hybridized local and charge transfer molecules rotation in excited state on exciton utilization. Sci. Rep. 11, 17686 (2021).
Hedin, L. New method for calculating the one-particle green’s function with application to the electron-gas problem. Phys. Rev. 139, A796–A823 (1965).
Martin, R. M., Reining, L. & Ceperley, D. M. Interacting Electrons: Theory and Computational Approaches (Cambridge University Press, 2016).
Rangel, T., Hamed, S. M., Bruneval, F. & Neaton, J. B. An assessment of low-lying excitation energies and triplet instabilities of organic molecules with an ab initio bethe-salpeter equation approach and the tamm-dancoff approximation. J. Chem. Phys. 146, 194108 (2017).
Jacquemin, D., Duchemin, I. & Blase, X. Benchmarking the bethe-salpeter formalism on a standard organic molecular set. J. Chem. Theory Comput. 11, 3290–3304 (2015).
Mansouri, M., Casanova, D., Koval, P. & Sánchez-Portal, D. GW approximation for open-shell molecules: a first-principles study. N. J. Phys. 23, 093027 (2021).
Mahmoodi, T. & Mansouri, M. Structural effects of substitutional impurities on MoO3 bilayers: a first principles study. J. Korean Phys. Soc. 69, 1439–1444 (2016).
Nitzan, A. & Ratner, M. A. Electron transport in molecular wire junctions. Science 300, 1384–1389 (2003).
Bruneval, F. et al. Molgw 1: many-body perturbation theory software for atoms, molecules, and clusters. Comput. Phys. Commun. 208, 149–161 (2016).
Giannozzi, P. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21, 395502 (2009).
Hamann, D. R. Optimized norm-conserving vanderbilt pseudopotentials. Phys. Rev. B 88, 085117 (2013).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (dft-d) for the 94 elements h-pu. J. Chem. Phys. 132, 154104 (2010).
Deslippe, J. et al. BerkeleyGW: a massively parallel computer package for the calculation of the quasiparticle and optical properties of materials and nanostructures. Comput. Phys. Commun. 183, 1269–1289 (2012).
Acknowledgements
We thank the Mare Nostrum Supercomputer of the Red Española de Supercomputación (BSC-RES) and the Centro de Computación Científica de la Universidad Autónoma de Madrid (CCC-UAM) for providing computational resources. This work has been supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 951224, TOMATTO), the Ministerio de Ciencia e Innovación MICINN (Spain) through the projects PID2022-138288NB-C31 and PID2022-138288NB-C33, and the “Severo Ochoa" Programme for Centres of Excellence in R&D (CEX2020-001039-S).
Author information
Authors and Affiliations
Contributions
M.M. performed the theoretical calculations, analyzed the data, and drafted the initial manuscript. J.A.-C. carried out the experiments under the supervision of N.M. All authors discussed the results. M.M., C.D., and F.M. revised the manuscript. F.M. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mansouri, M., Díaz, C., Alcolea-Cerdán, J.T. et al. Adsorption of organic donor-acceptor molecules on graphene/SiC preserves light-induced charge transfer. Commun Chem (2026). https://doi.org/10.1038/s42004-026-01943-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-026-01943-6