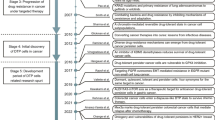
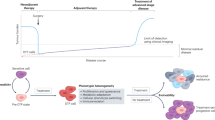
Abstract
Disease relapse driven by acquired drug resistance limits the effectiveness of most systemic anti-cancer agents. Targeting persistent cancer cells in residual disease before relapse has emerged as a potential strategy for enhancing the efficacy and the durability of current therapies. However, barriers remain to implementing persister-directed approaches in the clinic. This Perspective discusses current preclinical and clinical complexities and outlines key steps toward the development of clinical strategies that target therapy-persistent residual disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Turke, A. B. et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell 17, 77–88 (2010).
Su, K.-Y. et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J. Clin. Oncol. 30, 433–440 (2012).
Ye, X. et al. High T790M detection rate in TKI-naive NSCLC with EGFR sensitive mutation: truth or artifact? J. Thorac. Oncol. 8, 1118–1120 (2013).
Hata, A. N. et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat. Med. 22, 262–269 (2016).
Ramirez, M. et al. Diverse drug-resistance mechanisms can emerge from drug-tolerant cancer persister cells. Nat. Commun. 7, 10690 (2016).
Sharma, S. V. et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80 (2010).
Berger, A. J. et al. IRS1 phosphorylation underlies the non-stochastic probability of cancer cells to persist during EGFR inhibition therapy. Nat. Cancer 2, 1055–1070 (2021).
Sequist, L. V. et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 3, 75ra26 (2011).
Shi, H. et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 4, 80–93 (2014).
Marcoux, N. et al. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J. Clin. Oncol. 37, 278–285 (2019).
Tsai, Y. S. et al. Rapid idiosyncratic mechanisms of clinical resistance to KRAS G12C inhibition. J. Clin. Invest. 132, e155523 (2022).
Awad, M. M. et al. Acquired resistance to KRASG12C inhibition in cancer. N. Engl. J. Med. 384, 2382–2393 (2021).
Maynard, A. et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell 182, 1232–1251 (2020).
Vokes, N. I. et al. Concurrent TP53 mutations facilitate resistance evolution in EGFR-mutant lung adenocarcinoma. J. Thorac. Oncol. 17, 779–792 (2022).
Ryl, T. et al. Cell-cycle position of single MYC-driven cancer cells dictates their susceptibility to a chemotherapeutic drug. Cell Syst. 5, 237–250 (2017).
Hastings, J. F. et al. Memory of stochastic single-cell apoptotic signaling promotes chemoresistance in neuroblastoma. Sci. Adv. 9, eabp8314 (2023).
Singh, D. K. et al. Patterns of basal signaling heterogeneity can distinguish cellular populations with different drug sensitivities. Mol. Syst. Biol. 6, 369 (2010).
Shaffer, S. M. et al. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 546, 431–435 (2017).
Min, M. & Spencer, S. L. Spontaneously slow-cycling subpopulations of human cells originate from activation of stress-response pathways. PLoS Biol. 17, e3000178 (2019).
Sun, X. et al. Modulating environmental signals to reveal mechanisms and vulnerabilities of cancer persisters. Sci. Adv. 8, eabi7711 (2022).
Obenauf, A. C. et al. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature 520, 368–372 (2015).
Schmitt, M. et al. Colon tumour cell death causes mTOR dependence by paracrine P2X4 stimulation. Nature 612, 347–353 (2022).
Pu, Y. et al. Drug-tolerant persister cells in cancer: the cutting edges and future directions. Nat. Rev. Clin. Oncol. 20, 799–813 (2023).
Shen, S., Vagner, S. & Robert, C. Persistent cancer cells: the deadly survivors. Cell 183, 860–874 (2020).
Cabanos, H. F. & Hata, A. N. Emerging insights into targeted therapy-tolerant persister cells in cancer. Cancers 13, 2666 (2021).
Conti, G. D., Dias, M. H. & Bernards, R. Fighting drug resistance through the targeting of drug-tolerant persister cells. Cancers 13, 1118 (2021).
Mikubo, M., Inoue, Y., Liu, G. & Tsao, M.-S. Mechanism of drug tolerant persister cancer cells: the landscape and clinical implication for therapy. J. Thorac. Oncol. 16, 1798–1809 (2021).
Rambow, F. et al. Toward minimal residual disease-directed therapy in melanoma. Cell 174, 843–855 (2018).
Zhou, X. et al. Persister cell phenotypes contribute to poor patient outcomes after neoadjuvant chemotherapy in PDAC. Nat. Cancer 4, 1362–1381 (2023).
Isozaki, H. et al. Therapy-induced APOBEC3A drives evolution of persistent cancer cells. Nature 620, 393–401 (2023).
Russo, M. et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science 366, 1473–1480 (2019).
Goyal, Y. et al. Diverse clonal fates emerge upon drug treatment of homogeneous cancer cells. Nature 620, 651–659 (2023).
Guler, G. D. et al. Repression of stress-induced LINE-1 expression protects cancer cell subpopulations from lethal drug exposure. Cancer Cell 32, 221–237 (2017).
Hangauer, M. J. et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250 (2017).
Dhimolea, E. et al. An embryonic diapause-like adaptation with suppressed Myc activity enables tumor treatment persistence. Cancer Cell 39, 240–256 (2021).
Rehman, S. K. et al. Colorectal cancer cells enter a diapause-like DTP state to survive chemotherapy. Cell 184, 226–242 (2021).
Hu, H. et al. Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms. Cancer Cell 39, 1531–1547 (2021).
Kleczko, E. K. et al. Durable responses to alectinib in murine models of EML4-ALK lung cancer requires adaptive immunity. NPJ Precis. Oncol. 7, 15 (2023).
Sehgal, K. et al. Dynamic single-cell RNA sequencing identifies immunotherapy persister cells following PD-1 blockade. J. Clin. Invest. 131, e135038 (2020).
Biehs, B. et al. A cell identity switch allows residual BCC to survive Hedgehog pathway inhibition. Nature 562, 429–433 (2018).
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013).
Jordan, N. V. et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 537, 102–106 (2016).
Tian, J. et al. Combined PD-1, BRAF and MEK inhibition in BRAFV600E colorectal cancer: a phase 2 trial. Nat. Med. 29, 458–466 (2023).
Bao, F. et al. Integrative spatial analysis of cell morphologies and transcriptional states with MUSE. Nat. Biotechnol. 40, 1200–1209 (2022).
Pappas, L., Adalsteinsson, V. A. & Parikh, A. R. The emerging promise of liquid biopsies in solid tumors. Nat. Cancer 3, 1420–1422 (2022).
Gray, J. E. et al. Early clearance of plasma epidermal growth factor receptor mutations as a predictor of outcome on osimertinib in advanced non-small cell lung cancer; exploratory analysis from AURA3 and FLAURA. Clin. Cancer Res. 29, 3340–3351 (2023).
Paweletz, C. P. et al. Early changes in circulating cell-free KRAS G12C predict response to adagrasib in KRAS mutant non-small cell lung cancer patients. Clin. Cancer Res. 29, 3074–3080 (2023).
Tolmeijer, S. H. et al. Early on-treatment changes in circulating tumor DNA fraction and response to enzalutamide or abiraterone in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 29, 2835–2844 (2023).
Oxnard, G. R. et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J. Clin. Oncol. 34, 3375–3382 (2016).
Larson, M. H. et al. A comprehensive characterization of the cell-free transcriptome reveals tissue- and subtype-specific biomarkers for cancer detection. Nat. Commun. 12, 2357 (2021).
Roskams-Hieter, B. et al. Plasma cell-free RNA profiling distinguishes cancers from pre-malignant conditions in solid and hematologic malignancies. NPJ Precis. Oncol. 6, 28 (2022).
Esfahani, M. S. et al. Inferring gene expression from cell-free DNA fragmentation profiles. Nat. Biotechnol. 40, 585–597 (2022).
Shen, S. Y. et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 563, 579–583 (2018).
Berchuck, J. E. et al. Detecting neuroendocrine prostate cancer through tissue-informed cell-free DNA methylation analysis. Clin. Cancer Res. 28, 928–938 (2021).
Sadeh, R. et al. ChIP–seq of plasma cell-free nucleosomes identifies gene expression programs of the cells of origin. Nat. Biotechnol. 39, 586–598 (2021).
Fedyuk, V. et al. Multiplexed, single-molecule, epigenetic analysis of plasma-isolated nucleosomes for cancer diagnostics. Nat. Biotechnol. 41, 212–221 (2023).
Baca, S. C. et al. Liquid biopsy epigenomic profiling for cancer subtyping. Nat. Med. 29, 2737–2741 (2023).
Brito-Rocha, T., Constâncio, V., Henrique, R. & Jerónimo, C. Shifting the cancer screening paradigm: the rising potential of blood-based multi-cancer early detection tests. Cells 12, 935 (2023).
Vandekerckhove, O. et al. Liquid biopsy in early-stage lung cancer: current and future clinical applications. Cancers 15, 2702 (2023).
Gomez, D. R. et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 17, 1672–1682 (2016).
Gomez, D. R. et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J. Clin. Oncol. 37, 1558–1565 (2019).
Solomon, B. J. et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir. Med. 11, 354–366 (2023).
Gouda, M. A., Buschhorn, L., Schneeweiss, A., Wahida, A. & Subbiah, V. N-of-1 trials in cancer drug development. Cancer Discov. 13, 1301–1309 (2023).
Drilon, A. et al. SHP2 inhibition sensitizes diverse oncogene-addicted solid tumors to re-treatment with targeted therapy. Cancer Discov. 13, 1789–1801 (2023).
Acknowledgements
A.N.H. was supported by US National Institutes of Health (NIH) R01 CA249291, P50 CA265826, the Break Through Cancer foundation and the Ludwig Center at Harvard. X.S. was supported by the QB3 Postdoc Entrepreneurship Fellowship. S.J.A. was supported by the Mark Foundation for Cancer Research ASPIRE Award. L.F.W. was supported by NIH R01 CA184984.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
A.N.H. has received grants and/or research support from Amgen, BridgeBio, Bristol Myers Squibb, C4 Therapeutics, Eli Lilly, Novartis, Nuvalent, Pfizer and Scorpion Therapeutics and has served as a compensated consultant for Amgen, Engine Biosciences, Nuvalent, Oncovalent, Pfizer, TigaTx and Tolremo Therapeutics. X.S., L.F.W. and S.J.A. declare no competing interests.
Peer review information
Nature Cancer thanks Peter Bailey and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, X., Wu, L.F., Altschuler, S.J. et al. Targeting therapy-persistent residual disease. Nat Cancer 5, 1298–1304 (2024). https://doi.org/10.1038/s43018-024-00819-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-024-00819-9
This article is cited by
-
Drug-tolerant persister cells in cancer: bridging the gaps between bench and bedside
Nature Communications (2025)
-
Epigenetic reprogramming as the nexus of cancer stemness and therapy resistance: implications for biomarker discovery
Discover Oncology (2025)
-
Harnessing transcriptomics for discovery of natural products to overcome acquired cancer resistance
Archives of Pharmacal Research (2025)