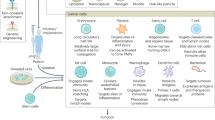
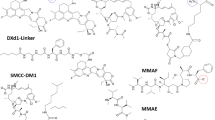
Abstract
Drug conjugates have emerged as promising tumor-targeted cytotoxics with an improved therapeutic index compared to classical chemotherapeutics. Although traditionally based on antibody ligands, high-throughput screening methods, such as peptide display and DNA-encoded chemical libraries, have enabled the isolation of ultra-high-affinity small ligands and the generation of drug conjugates with better tumor-targeting performance. This Perspective examines the history, major clinical milestones and future of drug conjugates for cancer treatment. We also discuss a new wave of combination modalities, linker strategies, and the development of conjugates based on large and small delivery vehicles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gilman, A. The initial clinical trial of nitrogen mustard. Am. J. Surg. 105, 574–578 (1963).
Camidge, R. The story of Taxol: nature and politics in the pursuit of an anti-cancer drug. BMJ 323, 115 (2001).
Van Cutsem, E. et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J. Clin. Oncol. 24, 4991–4997 (2006).
van der Veldt, A. A. M. et al. Biodistribution and radiation dosimetry of 11C-labelled docetaxel in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 37, 1950–1958 (2010).
Chari, R. V. J., Miller, M. L. & Widdison, W. C. Antibody–drug conjugates: an emerging concept in cancer therapy. Angew. Chem. Int. Ed. Engl. 53, 3796–3827 (2014).
Srinivasarao, M., Galliford, C. V. & Low, P. S. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat. Rev. Drug Discov. 14, 203–219 (2015).
Cazzamalli, S., Corso, A. D. & Neri, D. Targeted delivery of cytotoxic drugs: challenges, opportunities and new developments. Chimia (Aarau) 71, 712–715 (2017).
Lambert, J. M. & Berkenblit, A. Antibody–drug conjugates for cancer treatment. Annu. Rev. Med. 69, 191–207 (2018).
Köhler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Doronina, S. O. et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 21, 778–784 (2003).
Li, W. et al. Synthesis and evaluation of camptothecin antibody–drug conjugates. ACS Med. Chem. Lett. 10, 1386–1392 (2019).
Elmroth, K., Nygren, J., Mårtensson, S., Ismail, I. H. & Hammarsten, O. Cleavage of cellular DNA by calicheamicin γ1. DNA Repair (Amst.) 2, 363–374 (2003).
Tang, Y. et al. Real-time analysis on drug–antibody ratio of antibody–drug conjugates for synthesis, process optimization, and quality control. Sci. Rep. 7, 7763 (2017).
Sadowsky, J. D. et al. Development of efficient chemistry to generate site-specific disulfide-linked protein– and peptide–payload conjugates: application to THIOMAB antibody–drug conjugates. Bioconjug. Chem. 28, 2086–2098 (2017).
Stefan, N. et al. Highly potent, anthracycline-based antibody–drug conjugates generated by enzymatic, site-specific conjugation. Mol. Cancer Ther. 16, 879–892 (2017).
Senter, P. D. Potent antibody drug conjugates for cancer therapy. Curr. Opin. Chem. Biol. 13, 235–244 (2009).
Doronina, S. O. et al. Novel peptide linkers for highly potent antibody–auristatin conjugate. Bioconjug. Chem. 19, 1960–1963 (2008).
Polakis, P. Antibody drug conjugates for cancer therapy. Pharmacol. Rev. 68, 3–19 (2016).
Staudacher, A. H. & Brown, M. P. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br. J. Cancer 117, 1736–1742 (2017).
Kovtun, Y. V. et al. Antibody–drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 66, 3214–3221 (2006).
Demetri, G. D. et al. First-in-human phase I study of ABBV-085, an antibody–drug conjugate targeting LRRC15, in sarcomas and other advanced solid tumors. Clin. Cancer Res. 27, 3556–3566 (2021).
Fitzgerald, A. A. & Weiner, L. M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 39, 783–803 (2020).
Guo, Y. et al. Rational identification of novel antibody–drug conjugate with high bystander killing effect against heterogeneous tumors. Adv. Sci. (Weinh.) 11, e2306309 (2024).
Han, Y., Tian, X., Zhai, J. & Zhang, Z. Clinical application of immunogenic cell death inducers in cancer immunotherapy: turning cold tumors hot. Front. Cell Dev. Biol. 12, 1363121 (2024).
Denekamp, J. Vascular attack as a therapeutic strategy for cancer. Cancer Metastasis Rev. 9, 267–282 (1990).
Singh, A. P. & Shah, D. K. A “dual” cell-level systems PK–PD model to characterize the bystander effect of ADC. J. Pharm. Sci. 108, 2465–2475 (2019).
Zana, A. et al. Fibroblast activation protein triggers release of drug payload from non-internalizing small molecule drug conjugates in solid tumors. Clin. Cancer Res. 28, 5440–5454 (2022).
Cazzamalli, S., Dal Corso, A., Widmayer, F. & Neri, D. Chemically defined antibody– and small molecule–drug conjugates for in vivo tumor targeting applications: a comparative analysis. J. Am. Chem. Soc. 140, 1617–1621 (2018).
Bartlett, N. L. et al. Brentuximab vedotin activity in diffuse large B-cell lymphoma with CD30 undetectable by visual assessment of conventional immunohistochemistry. Leuk. Lymphoma 58, 1607–1616 (2017).
Goyal, A., Hordinsky, M. & Lazaryan, A. Impressive response of CD30-negative, treatment-refractory mycosis fungoides to brentuximab vedotin. Dermatol. Ther. 32, e12835 (2019).
Cahuzac, H. & Devel, L. Analytical methods for the detection and quantification of ADCs in biological matrices. Pharmaceuticals 13, 462 (2020).
Lamont, L. et al. Quantitative mass spectrometry imaging of drugs and metabolites: a multiplatform comparison. Anal. Bioanal. Chem. 413, 2779–2791 (2021).
Shastry, M. et al. Rise of antibody–drug conjugates: the present and future. Am. Soc. Clin. Oncol. Educ. Book 43, e390094 (2023).
Hamann, P. R. et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody–calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug. Chem. 13, 47–58 (2002).
Wei, A. H. & Tiong, I. S. Midostaurin, enasidenib, CPX-351, gemtuzumab ozogamicin, and venetoclax bring new hope to AML. Blood 130, 2469–2474 (2017).
Appelbaum, F. R. & Bernstein, I. D. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood 130, 2373–2376 (2017).
Fanale, M. A. et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin. Cancer Res. 18, 248–255 (2012).
Jagadeesh, D. et al. Response to brentuximab vedotin by CD30 expression in non-Hodgkin lymphoma. Oncologist 27, 864–873 (2022).
Cabel, L. et al. TOPOLOGY: a phase II study to evaluate the efficacy and toxicities of PLX038, in patients with locally advanced or metastatic triple-negative breast cancer. J. Clin. Oncol. 42, TPS1142 (2024).
Oflazoglu, E. et al. Macrophages contribute to the antitumor activity of the anti-CD30 antibody SGN-30. Blood 110, 4370–4372 (2007).
Lewis Phillips, G. D. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008).
Perez, E. A. et al. Relationship between HER2 expression and efficacy with first-line trastuzumab emtansine compared with trastuzumab plus docetaxel in TDM4450g: a randomized phase II study of patients with previously untreated HER2-positive metastatic breast cancer. Breast Cancer Res. 16, R50 (2014).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. In 50 Landmark Papers Every Breast Surgeon Should Know (eds Wyld, L. et al.) Ch. 38 (CRC, 2024).
Kantarjian, H. et al. Inotuzumab ozogamicin, an anti-CD22–calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 13, 403–411 (2012).
Kantarjian, H. M. et al. Inotuzumab ozogamicin for relapsed/refractory acute lymphoblastic leukemia in the INO-VATE trial: CD22 pharmacodynamics, efficacy, and safety by baseline CD22. Clin. Cancer Res. 27, 2742–2754 (2021).
Wintering, A. et al. CD22low/Bcl-2high expression identifies poor response to inotuzumab ozogamicin in relapsed/refractory acute lymphoblastic leukemia. Blood Adv. 7, 251–255 (2023).
DeAngelo, D. J. et al. Inotuzumab ozogamicin for relapsed/refractory acute lymphoblastic leukemia: outcomes by disease burden. Blood Cancer J. 10, 81 (2020).
Cortés, J. et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386, 1143–1154 (2022).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Meric-Bernstam, F. et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J. Clin. Oncol. 42, 47–58 (2024).
Yu, E. Y. et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 22, 872–882 (2021).
Wahby, S. et al. FDA approval summary: accelerated approval of sacituzumab govitecan-hziy for third-line treatment of metastatic triple-negative breast cancer. Clin. Cancer Res. 27, 1850–1854 (2021).
Santi, D. V., Cabel, L. & Bidard, F.-C. Does sacituzumab-govitecan act as a conventional antibody drug conjugate (ADC), a prodrug of SN-38 or both? Ann. Transl. Med. 9, 1113 (2021).
Lewis, G. D. et al. The HER2-directed antibody–drug conjugate DHES0815A in advanced and/or metastatic breast cancer: preclinical characterization and phase 1 trial results. Nat. Commun. 15, 466 (2024).
Chooniedass, S. et al. DeBouganin diabody fusion protein overcomes drug resistance to ADCs comprised of anti-microtubule agents. Molecules 21, 1741 (2016).
Bardia, A. et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann. Oncol. 32, 1148–1156 (2021).
Gray, J. E. et al. Therapy of small cell lung cancer (SCLC) with a topoisomerase-I-inhibiting antibody–drug conjugate (ADC) targeting Trop-2, sacituzumab govitecan. Clin. Cancer Res. 23, 5711–5719 (2017).
Santi, D. V., Ashley, G. W., Cabel, L. & Bidard, F.-C. Could a long-acting prodrug of SN-38 be efficacious in sacituzumab govitecan-resistant tumors? BioDrugs 38, 171–176 (2024).
Vergote, I. et al. Tisotumab vedotin as second- or third-line therapy for recurrent cervical cancer. N. Engl. J. Med. 391, 44–55 (2024).
Matulonis, U. A. et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J. Clin. Oncol. 41, 2436–2445 (2023).
Lonial, S. et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 21, 207–221 (2020).
Tilly, H. et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N. Engl. J. Med. 386, 351–363 (2022).
Wadleigh, M. et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood 102, 1578–1582 (2003).
Kantarjian, H. M. et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N. Engl. J. Med. 375, 740–753 (2016).
Nguyen, T. D., Bordeau, B. M. & Balthasar, J. P. Mechanisms of ADC toxicity and strategies to increase ADC tolerability. Cancers (Basel) 15, 713 (2023).
Nolting, B. Linker technologies for antibody–drug conjugates. Methods Mol. Biol. 1045, 71–100 (2013).
Zhang, D. et al. Catalytic cleavage of disulfide bonds in small molecules and linkers of antibody–drug conjugates. Drug Metab. Dispos. 47, 1156–1163 (2019).
Migliorini, F. et al. A pH-responsive crosslinker platform for antibody–drug conjugate (ADC) targeting delivery. Chem. Commun. (Camb.) 58, 10532–10535 (2022).
Jeffrey, S. C. et al. Minor groove binder antibody conjugates employing a water soluble β-glucuronide linker. Bioorg. Med. Chem. Lett. 17, 2278–2280 (2007).
Dubowchik, G. M. et al. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug. Chem. 13, 855–869 (2002).
Caculitan, N. G. et al. Cathepsin B is dispensable for cellular processing of cathepsin B-cleavable antibody–drug conjugates. Cancer Res. 77, 7027–7037 (2017).
Dorywalska, M. et al. Molecular basis of valine–citrulline–PABC linker instability in site-specific ADCs and its mitigation by linker design. Mol. Cancer Ther. 15, 958–970 (2016).
Fu, Z., Li, S., Han, S., Shi, C. & Zhang, Y. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct. Target. Ther. 7, 93 (2022).
Bargh, J. D., Isidro-Llobet, A., Parker, J. S. & Spring, D. R. Cleavable linkers in antibody–drug conjugates. Chem. Soc. Rev. 48, 4361–4374 (2019).
Choi, K. Y., Swierczewska, M., Lee, S. & Chen, X. Protease-activated drug development. Theranostics 2, 156–178 (2012).
Backhaus, P. et al. Translational imaging of the fibroblast activation protein (FAP) using the new ligand [68Ga]Ga-OncoFAP-DOTAGA. Eur. J. Nucl. Med. Mol. Imaging 49, 1822–1832 (2022).
Chen, I.-J. et al. Selective antibody activation through protease-activated pro-antibodies that mask binding sites with inhibitory domains. Sci. Rep. 7, 11587 (2017).
Rossin, R. et al. Chemically triggered drug release from an antibody–drug conjugate leads to potent antitumour activity in mice. Nat. Commun. 9, 1484 (2018).
Fu, C. et al. Peptide–drug conjugates (PDCs): a novel trend of research and development on targeted therapy, hype or hope? Acta Pharm. Sin. 13, 498–516 (2023).
Srinivasarao, M. & Low, P. S. Ligand-targeted drug delivery. Chem. Rev. 117, 12133–12164 (2017).
Dennis, M. S. et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 67, 254–261 (2007).
Jones, P. T., Dear, P. H., Foote, J., Neuberger, M. S. & Winter, G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321, 522–525 (1986).
McCafferty, J., Griffiths, A. D., Winter, G. & Chiswell, D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348, 552–554 (1990).
Tamura, K. et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 20, 816–826 (2019).
Shuch, B. et al. [89Zr]Zr-girentuximab for PET–CT imaging of clear-cell renal cell carcinoma: a prospective, open-label, multicentre, phase 3 trial. Lancet Oncol. 25, 1277–1287 (2024).
Carter, P. J. & Rajpal, A. Designing antibodies as therapeutics. Cell 185, 2789–2805 (2022).
Carter, P. J. Introduction to current and future protein therapeutics: a protein engineering perspective. Exp. Cell Res. 317, 1261–1269 (2011).
Ma, X., Wang, M., Ying, T. & Wu, Y. Reforming solid tumor treatment: the emerging potential of smaller format antibody–drug conjugate. Antib. Ther. 7, 114–122 (2024).
Borsi, L. et al. Selective targeting of tumoral vasculature: comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int. J. Cancer 102, 75–85 (2002).
Ongaro, T. et al. A novel anti-cancer L19–interleukin-12 fusion protein with an optimized peptide linker efficiently localizes in vivo at the site of tumors. J. Biotechnol. 291, 17–25 (2019).
Schmidt, M. M. & Wittrup, K. D. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol. Cancer Ther. 8, 2861–2871 (2009).
Takatsuji, R. et al. Ribosomal synthesis of backbone-cyclic peptides compatible with in vitro display. J. Am. Chem. Soc. 141, 2279–2287 (2019).
Heinis, C., Rutherford, T., Freund, S. & Winter, G. Phage-encoded combinatorial chemical libraries based on bicyclic peptides. Nat. Chem. Biol. 5, 502–507 (2009).
Bashir, B. et al. Results from first-in-human phase I dose-escalation study of a novel bicycle toxin conjugate targeting EphA2 (BT5528) in patients with advanced solid tumors. J. Clin. Oncol. 42, 3443–3452 (2024).
Fakiri, M. E. et al. Development and preclinical characterization of a novel radiotheranostic EphA2-targeting bicyclic peptide. Theranostics 14, 4701–4712 (2024).
Baum, R. P. et al. Feasibility, biodistribution, and preliminary dosimetry in peptide-targeted radionuclide therapy of diverse adenocarcinomas using 177Lu-FAP-2286: first-in-humans results. J. Nucl. Med. 63, 415–423 (2022).
Hofman, M. S. et al. First-in-human safety, imaging, and dosimetry of a carbonic anhydrase IX-targeting peptide, [68Ga]Ga-DPI-4452, in patients with clear cell renal cell carcinoma. J. Nucl. Med. 65, 740–743 (2024).
Poli, G. L. et al. Radretumab radioimmunotherapy in patients with brain metastasis: a 124I-L19SIP dosimetric PET study. Cancer Immunol. Res. 1, 134–143 (2013).
Wei, X., Schlenkhoff, C., Schwarz, B., Essler, M. & Ahmadzadehfar, H. Combination of 177Lu-PSMA-617 and external radiotherapy for the treatment of cerebral metastases in patients with castration-resistant metastatic prostate cancer. Clin. Nucl. Med. 42, 704–706 (2017).
Millul, J. et al. An ultra-high-affinity small organic ligand of fibroblast activation protein for tumor-targeting applications. Proc. Natl Acad. Sci. USA 118, e2101852118 (2021).
Puglioli, S. et al. Selective tumor targeting enabled by picomolar fibroblast activation protein inhibitors isolated from a DNA-encoded affinity maturation library. Chem 9, 411–429 (2023).
Vergote, I. & Leamon, C. P. Vintafolide: a novel targeted therapy for the treatment of folate receptor expressing tumors. Ther. Adv. Med. Oncol. 7, 206–218 (2015).
Gerber, H. P., Sapra, P., Loganzo, F. & May, C. Combining antibody–drug conjugates and immune-mediated cancer therapy: what to expect? Biochem. Pharmacol. 102, 1–6 (2016).
Bauzon, M. et al. Maytansine-bearing antibody–drug conjugates induce in vitro hallmarks of immunogenic cell death selectively in antigen-positive target cells. Oncoimmunology 8, e1565859 (2019).
Powles, T. et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N. Engl. J. Med. 390, 875–888 (2024).
Goto, Y. et al. TROPION-Lung02: datopotamab deruxtecan (Dato-DXd) plus pembrolizumab (pembro) with or without platinum chemotherapy (Pt-CT) in advanced non-small cell lung cancer (aNSCLC). J. Clin. Oncol. 41, 9004 (2023).
Natangelo, S. et al. Radiation therapy, tissue radiosensitization, and potential synergism in the era of novel antibody–drug conjugates. Crit. Rev. Oncol. Hematol. 195, 104270 (2024).
Hingorani, D. V. et al. Precision chemoradiotherapy for HER2 tumors using antibody conjugates of an auristatin derivative with reduced cell permeability. Mol. Cancer Ther. 19, 157–167 (2020).
Wei, Q. et al. The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors. J. Hematol.Oncol. 17, 1 (2024).
Koide, Y. & Kodaira, T. Concurrent antibody–drug conjugates and radiotherapy: a new perspective on radiation necrosis in HER2-positive breast cancer brain metastases from the DESTINY-Breast03 and HER2CLIMB trials. ESMO Open 9, 103620 (2024).
Salvestrini, V. et al. Safety profile of trastuzumab-emtansine (T-DM1) with concurrent radiation therapy: a systematic review and meta-analysis. Radiother. Oncol. 186, 109805 (2023).
Sgouros, G., Bodei, L., McDevitt, M. R. & Nedrow, J. R. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat. Rev. Drug Discov. 19, 589–608 (2020).
Pretelli, G., Mati, K., Motta, L. & Stathis, A. Antibody–drug conjugates combinations in cancer treatment. Explor. Target. Antitumor Ther. 5, 714–741 (2024).
Gross, S. M. et al. Analysis and modeling of cancer drug responses using cell cycle phase-specific rate effects. Nat. Commun. 14, 3450 (2023).
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
O’Malley, D. M. et al. Mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody–drug conjugate (ADC), in combination with bevacizumab in patients (pts) with platinum-resistant ovarian cancer: final findings from the FORWARD II study. J. Clin. Oncol. 37, 5520 (2019).
Boswell, C. A. et al. Effects of anti-VEGF on predicted antibody biodistribution: roles of vascular volume, interstitial volume, and blood flow. PLoS ONE 6, e17874 (2011).
Halin, C. et al. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor α. Cancer Res. 63, 3202–3210 (2003).
Thurber, G. M. et al. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat. Commun. 4, 1504 (2013).
Zana, A. et al. A comparative analysis of fibroblast activation protein-targeted small molecule–drug, antibody–drug, and peptide–drug conjugates. Bioconjug. Chem. 34, 1205–1211 (2023).
van der Veldt, A. A. M. et al. Toward prediction of efficacy of chemotherapy: a proof of concept study in lung cancer patients using [11C]docetaxel and positron emission tomography. Clin. Cancer Res. 19, 4163–4173 (2013).
Oehler, S. et al. A DNA-encoded chemical library based on chiral 4-amino-proline enables stereospecific isozyme-selective protein recognition. Nat. Chem. 15, 1431–1443 (2023).
Loganzo, F., Sung, M. & Gerber, H.-P. Mechanisms of resistance to antibody–drug conjugates. Mol. Cancer Ther. 15, 2825–2834 (2016).
Chen, M. et al. Optimal sequential strategies for antibody–drug conjugate in metastatic breast cancer: evaluating efficacy and cross-resistance. Oncologist 29, e957–e966 (2024).
Verhoeff, S. R. et al. [89Zr]Zr-DFO-girentuximab and [18F]FDG PET/CT to predict watchful waiting duration in patients with metastatic clear-cell renal cell carcinoma. Clin. Cancer Res. 29, 592–601 (2023).
Vegt, E. et al. Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J. Nucl. Med. 51, 1049–1058 (2010).
Acknowledgements
We thank G. Rotta for the support in preparing the original figures. The preparation of this article was entirely funded by Philochem AG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.N. is the cofounder, CEO, CSO and President of the Scientific Advisory Board of Philogen. S.C. and E.P. are employed by Philochem AG, the research and development unit of the Philogen Group.
Peer review
Peer review information
Nature Cancer thanks Sara Hurvitz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cazzamalli, S., Puca, E. & Neri, D. Past, present and future of drug conjugates for cancer therapy. Nat Cancer 6, 1494–1504 (2025). https://doi.org/10.1038/s43018-025-01042-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-025-01042-w