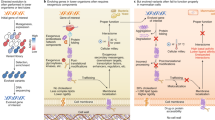
Abstract
Directed evolution has revolutionized biomolecular engineering by applying cycles of mutation, amplification and selection to genes of interest (GOIs). However, classical directed evolution methods that rely on manually staged evolutionary cycles constrain the scale and depth of the evolutionary search that is possible. We describe genetic systems that achieve cycles of rapid mutation, amplification and selection fully inside living cells, enabling the continuous evolution of GOIs as cells grow. These systems advance the scale, evolutionary search depth, ease and overall power of directed evolution and access important new areas of protein evolution and engineering.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Arnold, F. H. Design by directed evolution. Acc. Chem. Res. 31, 125–131 (1998).
Packer, M. S. & Liu, D. R. Methods for the directed evolution of proteins. Nat. Rev. Genet. 16, 379–394 (2015).
Drake, J. W., Charlesworth, B., Charlesworth, D. & Crow, J. F. Rates of spontaneous mutation. Genetics 148, 1667–1686 (1998).
Chen, K. & Arnold, F. H. Engineering new catalytic activities in enzymes. Nat. Catal. 3, 203–213 (2020).
Esvelt, K. M., Carlson, J. C. & Liu, D. R. A system for the continuous directed evolution of biomolecules. Nature 472, 499–503 (2011).
Miller, S. M., Wang, T. & Liu, D. R. Phage-assisted continuous and non-continuous evolution. Nat. Protoc. 15, 4101–4127 (2020).
Morrison, M. S., Podracky, C. J. & Liu, D. R. The developing toolkit of continuous directed evolution. Nat. Chem. Biol. 16, 610–619 (2020).
Hendel, S. J. & Shoulders, M. D. Directed evolution in mammalian cells. Nat. Methods. 18, 346–357 (2021).
Fabret, C. et al. Efficient gene targeted random mutagenesis in genetically stable Escherichia coli strains. Nucleic Acids Res. 28, 95 (2000).
Camps, M., Naukkarinen, J., Johnson, B. P. & Loeb, L. A. Targeted gene evolution in Escherichia coli using a highly error-prone DNA polymerase I. Proc. Natl Acad. Sci. USA 100, 9727–9732 (2003).
Finney-Manchester, S. P. & Maheshri, N. Harnessing mutagenic homologous recombination for targeted mutagenesis in vivo by TaGTEAM. Nucleic Acids Res. 41, 1–10 (2013).
Crook, N. et al. In vivo continuous evolution of genes and pathways in yeast. Nat. Commun. 7, 13051 (2016).
Ravikumar, A., Arrieta, A. & Liu, C. C. An orthogonal DNA replication system in yeast. Nat. Chem. Biol. 10, 175–177 (2014). This work establishes an orthogonal DNA replication system in yeast that enables the elevation of mutation rates on an orthogonal plasmid replicated by a cognate orthogonal error-prone DNAP.
Hess, G. T. et al. Directed evolution using dCas9-targeted somatic hypermutation in mammalian cells. Nat. Methods. 13, 1036–1042 (2016). This work is an early demonstration that attachment of mutagenic machinery, in this case a cytidine deaminase, to dCas9 is an effective strategy for targeting hypermutation to desired loci in mammalian cells.
Ma, Y. et al. Targeted AID-mediated mutagenesis (TAM) enables efficient genomic diversification in mammalian cells. Nat. Methods. 13, 1029–1035 (2016).
Moore, C. L., Papa, L. J. & Shoulders, M. D. A processive protein chimera introduces mutations across defined DNA regions in vivo. J. Am. Chem. Soc. 140, 11560–11564 (2018). This work establishes the MutaT7 strategy involving the fusion of a DNA-damaging cytidine deaminase to a processive RNA polymerase to achieve in vivo targeted hypermutation of multi-kilobyte DNA sequences, thereby enabling continuous evolution of GOIs inside E. coli.
Halperin, S. O. et al. CRISPR-guided DNA polymerases enable diversification of all nucleotides in a tunable window. Nature. 560, 248–252 (2018). This work demonstrates that fusion of an error-prone DNAP to nCas9 achieves hypermutation at desired gRNA-targeted loci in E. coli to support continuous in vivo diversification and evolution of GOIs.
Ravikumar, A., Arzumanyan, G. A., Obadi, M. K. A., Javanpour, A. A. & Liu, C. C. Scalable, continuous evolution of genes at mutation rates above genomic error thresholds. Cell. 175, 1946–1957.e13 (2018). This work establishes a highly error-prone orthogonal DNA replication system that durably hypermutates an orthogonal plasmid at mutation rates 100,000-fold higher than the genome in yeast, thereby supporting the continuous evolution of GOIs for extended periods of time and at scale, as demonstrated through the replicate evolution of drug resistance by a malarial drug target.
Yi, X., Khey, J., Kazlauskas, R. J. & Travisano, M. Plasmid hypermutation using a targeted artificial DNA replisome. Sci. Adv. 7, eabg871 (2021).
Yi, X., Kazlauskas, R. & Travisano, M. Evolutionary innovation using EDGE, a system for localized elevated mutagenesis. PLoS ONE 15, 1–18 (2020).
Jensen, E. D. et al. A synthetic RNA-mediated evolution system in yeast. Nucleic Acids Res. 49, 1–12 (2021).
Chen, H. et al. Efficient, continuous mutagenesis in human cells using a pseudo-random DNA editor. Nat. Biotechnol. 38, 165–168 (2020). This work presents an extension of the MutaT7 strategy to mammalian cells, enabling the continuous targeted hypermutation and evolution of GOIs inside human cells.
Álvarez, B., Mencía, M., de Lorenzo, V. & Fernández, L. Á. In vivo diversification of target genomic sites using processive base deaminase fusions blocked by dCas9. Nat. Commun. 11, 6436 (2020). This work expands the MutaT7 technology through the fusion of new base deaminases to T7RNAP (thereby achieving targeted hypermutation with expanded mutational parameters in E. coli) and the addition of dCas9 to terminate polymerization by T7RNAP (thereby providing more control over the window of hypermutation).
Park, H. & Kim, S. Gene-specific mutagenesis enables rapid continuous evolution of enzymes in vivo. Nucleic Acids Res. 49, e32–e32 (2021). This work presents an expansion of MutaT7 technology to achieve exceptionally high rates of hypermutation in E. coli.
Cravens, A., Jamil, O. K., Kong, D., Sockolosky, J. T. & Smolke, C. D. Polymerase-guided base editing enables in vivo mutagenesis and rapid protein engineering. Nat. Commun. 12, 1579 (2021). This work extends the MutaT7 strategy to yeast, enabling the continuous targeted hypermutation and in vivo continuous evolution of GOIs in S. cerevisiae.
Butt, H., Ramirez, J. L. M. & Mahfouz, M. Synthetic evolution of herbicide resistance using a T7 RNAP-based random DNA base editor. Preprint at bioRxiv https://doi.org/10.1101/2021.11.30.470689 (2021).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 337, 816–821 (2012).
Tou, C. J., Schaffer, D. V. & Dueber, J. E. Targeted diversification in the S. cerevisiae genome with CRISPR-guided DNA polymerase i. ACS Synth. Biol. 9, 1911–1916 (2020).
Khanal, A., McLoughlin, S. Y., Kershner, J. P. & Copley, S. D. Differential effects of a mutation on the normal and promiscuous activities of orthologs: implications for natural and directed evolution. Mol. Biol. Evol. 32, 100–108 (2015).
Zheng, J., Payne, J. L. & Wagner, A. Cryptic genetic variation accelerates evolution by opening access to diverse adaptive peaks. Science 365, 347–353 (2019).
Gupta, R. D. & Tawfik, D. S. Directed enzyme evolution via small and effective neutral drift libraries. Nat. Methods. 5, 939–942 (2008).
Salverda, M. L. M. et al. Initial mutations direct alternative pathways of protein evolution. PLoS Genet. 7, e1001321 (2011).
Baier, F. et al. Cryptic genetic variation shapes the adaptive evolutionary potential of enzymes. eLife 8, 1–20 (2019).
Lang, G. I. et al. Pervasive genetic hitchhiking and clonal interference in forty evolving yeast populations. Nature. 500, 571–574 (2013).
Eigen, M., McCaskill, J. & Schuster, P. Molecular quasi-species. J. Phys. Chem. 92, 6881–6891 (1988).
Rix, G. & Liu, C. C. Systems for in vivo hypermutation: a quest for scale and depth in directed evolution. Curr. Opin. Chem. Biol. 64, 20–26 (2021). This work outlines the value of in vivo continuous evolution systems in accessing new categories of directed evolution experiments characterized by depth and scale.
Gunge, N. & Sakaguchi, K. Intergeneric transfer of deoxyribonucleic acid killer plasmids, pGKl1 and pGKl2, from Kluyveromyces lactis into Saccharomyces cerevisiae by cell fusion. J. Bacteriol. 147, 155–160 (1981).
Arzumanyan, G. A., Gabriel, K. N., Ravikumar, A., Javanpour, A. A. & Liu, C. C. Mutually orthogonal DNA replication systems in vivo. ACS Synth. Biol. 7, 1722–1729 (2018).
Zhong, Z., Ravikumar, A. & Liu, C. C. Tunable expression systems for orthogonal DNA replication. ACS Synth. Biol. 7, 2930–2934 (2018).
Kämper, J., Esser, K., Gunge, N. & Meinhardt, F. Heterologous gene expression on the linear DNA killer plasmid from Kluyveromyces lactis. Curr. Genet. 19, 109–118 (1991).
Javanpour, A. A. & Liu, C. C. Genetic compatibility and extensibility of orthogonal replication. ACS Synth. Biol. 8, 1249–1256 (2019).
Chamberlin, M., Mcgrath, J. & Waskell, L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 228, 227–231 (1970).
Tabor, S. & Richardson, C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl Acad. Sci. USA 82, 1074–1078 (1985).
McAllister, W. T., Morris, C., Rosenberg, A. H. & Studier, F. W. Utilization of bacteriophage T7 late promoters in recombinant plasmids during infection. J. Mol. Biol. 153, 527–544 (1981).
Thiel, V., Herold, J., Schelle, B. & Siddell, S. G. Infectious RNA transcribed in vitro from a cDNA copy of the human coronavirus genome cloned in vaccinia virus. J. Gen. Virol. 82, 1273–1281 (2001).
Steitz, T. A. The structural changes of T7 RNA polymerase from transcription initiation to elongation. Curr. Opin. Struct. Biol. 19, 683–690 (2009).
Conticello, S. G. The AID/APOBEC family of nucleic acid mutators. Genome Biol. 9, 229 (2008).
Gerber, A. P. & Keller, W. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science 286, 1146–1149 (1999).
Harris, R. S., Petersen-Mahrt, S. K. & Neuberger, M. S. RNA editing enzyme APOBEC1 and some of its homologs can act as DNA mutators. Mol. Cell. 10, 1247–1253 (2002).
Navaratnam, N. & Sarwar, R. An overview of cytidine deaminases. Int. J. Hematol. 83, 195–200 (2006).
Cacciamani, T. et al. Purification of human cytidine deaminase: molecular and enzymatic characterization and inhibition by synthetic pyrimidine analogs. Arch. Biochem. Biophys. 290, 285–292 (1991).
Chung, S. J., Fromme, J. C. & Verdine, G. L. Structure of human cytidine deaminase bound to a potent inhibitor. J. Med. Chem. 48, 658–660 (2005).
Lada, A. G. et al. Mutator effects and mutation signatures of editing deaminases produced in bacteria and yeast. Biochem 76, 131–146 (2011).
Vik, E. S. et al. Endonuclease V cleaves at inosines in RNA. Nat. Commun. 4, 2271 (2013).
Krokan, H. E., Drabløs, F. & Slupphaug, G. Uracil in DNA — occurrence, consequences and repair. Oncogene 21, 8935–8948 (2002).
Alseth, I., Dalhus, B. & Bjørås, M. Inosine in DNA and RNA. Curr. Opin. Genet. Dev. 26, 116–123 (2014).
Hirano, K. I., Min, J., Funahashi, T. & Davidson, N. O. Cloning and characterization of the rat apobec-1 gene: a comparative analysis of gene structure and promoter usage in rat and mouse. J. Lipid Res. 38, 1103–1119 (1997).
MacGinnitie, A. J., Anant, S. & Davidson, N. O. Mutagenesis of apobec-1, the catalytic subunit of the mammalian apolipoprotein B mRNA editing enzyme, reveals distinct domains that mediate cytosine nucleoside deaminase, RNA binding, and RNA editing activity. J. Biol. Chem. 270, 14768–14775 (1995).
Scott, J., Navaratnam, N., Bhattacharya, S. & Morrison, J. R. The apolipoprotein B messenger RNA editing enzyme. Curr. Opin. Lipidol. 5, 87–93 (1994).
Arakawa, H., HauschiLd, J. & Buerstedde, J. M. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science 295, 1301–1306 (2002).
Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 102, 553–563 (2000).
Rogozin, I. B. et al. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID–APOBEC family cytosine deaminase. Nat. Immunol. 8, 647–656 (2007).
Kim, J. et al. Structural and kinetic characterization of Escherichia coli TadA, the wobble-specific tRNA deaminase. Biochemistry. 45, 6407–6416 (2006).
Gaudelli, N. M. et al. Programmable base editing of T to G C in genomic DNA without DNA cleavage. Nature. 551, 464–471 (2017).
Richter, M. F. et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 38, 883–891 (2020).
Martínez-Salas, E. Internal ribosome entry site biology and its use in expression vectors. Curr. Opin. Biotechnol. 10, 458–464 (1999).
Wang, Z. & Mosbaugh, D. W. Uracil-DNA glycosylase inhibitor of bacteriophage PBS2: cloning and effects of expression of the inhibitor gene in Escherichia coli. J. Bacteriol. 170, 1082–1091 (1988).
Wang, Z. & Mosbaugh, D. W. Uracil-DNA glycosylase inhibitor gene of bacteriophage PBS2 encodes a binding protein specific for uracil-DNA glycosylase. J. Biol. Chem. 264, 1163–1171 (1989).
Karran, P., Cone, R. & Friedberg, E. C. Specificity of the bacteriophage PBS2 induced inhibitor of uracil-DNA glycosylase. Biochemistry. 20, 6092–6096 (1981).
Bennett, S. E. & Mosbaugh, D. W. Characterization of the Escherichia coli uracil-DNA glycosylase·inhibitor protein complex. J. Biol. Chem. 267, 22512–22521 (1992).
Bennett, S. E., Schimerlik, M. I. & Mosbaugh, D. W. Kinetics of the uracil-DNA glycosylase/inhibitor protein association. Ung interaction with Ugi, nucleic acids, and uracil compounds. J. Biol. Chem. 268, 26879–26885 (1993).
Wang, Y. et al. A novel strategy to engineer DNA polymerases for enhanced processivity and improved performance in vitro. Nucleic Acids Res. 32, 1197–1207 (2004).
Tizei, P. A. G., Csibra, E., Torres, L. & Pinheiro, V. B. Selection platforms for directed evolution in synthetic biology. Biochem. Soc. Trans. 44, 1165–1175 (2016).
Wang, Y. et al. Directed evolution: methodologies and applications. Chem. Rev. 121, 12384–12444 (2021).
Dickinson, B. C., Leconte, A. M., Allen, B., Esvelt, K. M. & Liu, D. R. Experimental interrogation of the path dependence and stochasticity of protein evolution using phage-assisted continuous evolution. Proc. Natl Acad. Sci. USA 110, 9007–9012 (2013).
Wang, L., Brock, A., Herberich, B. & Schultz, P. G. Expanding the genetic code of Escherichia coli. Science. 292, 498–500 (2001).
Carlson, J. C., Badran, A. H., Guggiana-Nilo, D. A. & Liu, D. R. Negative selection and stringency modulation in phage-assisted continuous evolution. Nat. Chem. Biol. 10, 216–222 (2014).
Blum, T. R. et al. Phage-assisted evolution of botulinum neurotoxin proteases with reprogrammed specificity. Science. 371, 803–810 (2021).
Szendro, I. G., Franke, J., De Visser, J. A. G. M. & Krug, J. Predictability of evolution depends nonmonotonically on population size. Proc. Natl Acad. Sci. USA 110, 571–576 (2013).
Salverda, M. L. M., Koomen, J., Koopmanschap, B., Zwart, M. P. & de Visser, J. A. G. M. Adaptive benefits from small mutation supplies in an antibiotic resistance enzyme. Proc. Natl Acad. Sci. USA 114, 12773–12778 (2017).
Steinberg, B. & Ostermeier, M. Environmental changes bridge evolutionary valleys. Sci. Adv. 2, e1500921 (2016).
Wong, B. G., Mancuso, C. P., Kiriakov, S., Bashor, C. J. & Khalil, A. S. Precise, automated control of conditions for high-throughput growth of yeast and bacteria with eVOLVER. Nat. Biotechnol. 36, 614–623 (2018).
Zhong, Z. et al. Automated continuous evolution of proteins in vivo. ACS Synth. Biol. 9, 1270–1276 (2020).
DeBenedictis, E. A. et al. Systematic molecular evolution enables robust biomolecule discovery. Nat. Methods. 19, 55–64 (2022).
Marks, D. S. et al. Protein 3D structure computed from evolutionary sequence variation. PLoS ONE 6, e28766 (2011).
Stiffler, M. A. et al. Protein structure from experimental evolution. Cell Syst. 10, 15–24.e5 (2020).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Yang, K. K., Wu, Z. & Arnold, F. H. Machine-learning-guided directed evolution for protein engineering. Nat. Methods. 16, 687–694 (2019).
Wu, Z., Kan, S. B. J., Lewis, R. D., Wittmann, B. J. & Arnold, F. H. Machine learning-assisted directed protein evolution with combinatorial libraries. Proc. Natl Acad. Sci. USA 116, 8852–8858 (2019).
Carr, I. M. et al. Inferring relative proportions of DNA variants from sequencing electropherograms. Bioinformatics 25, 3244–3250 (2009).
Shen, M. W., Zhao, K. T. & Liu, D. R. Reconstruction of evolving gene variants and fitness from short sequencing reads. Nat. Chem. Biol. 17, 1188–1198 (2021).
Goodwin, S., McPherson, J. D. & McCombie, W. R. Coming of age: ten years of next-generation sequencing technologies. Nat. Rev. Genet. 17, 333–351 (2016).
Amarasinghe, S. L. et al. Opportunities and challenges in long-read sequencing data analysis. Genome Biol. 21, 1–16 (2020).
Ravi, R. K., Walton, K. & Khosroheidari, M. in Disease Gene Identification: Methods and Protocols (ed. DiStefano, J. K.) 223–232 (Springer, 2018).
Stoler, N. & Nekrutenko, A. Sequencing error profiles of Illumina sequencing instruments. NAR. Genomics Bioinforma. 3, 1–9 (2021).
Wang, Y., Zhao, Y., Bollas, A., Wang, Y. & Au, K. F. Nanopore sequencing technology, bioinformatics and applications. Nat. Biotechnol. 39, 1348–1365 (2021).
Karst, S. M. et al. High-accuracy long-read amplicon sequences using unique molecular identifiers with Nanopore or PacBio sequencing. Nat. Methods. 18, 165–169 (2021).
Wenger, A. M. et al. Accurate circular consensus long-read sequencing improves variant detection and assembly of a human genome. Nat. Biotechnol. 37, 1155–1162 (2019).
Zurek, P. J., Knyphausen, P., Neufeld, K., Pushpanath, A. & Hollfelder, F. UMI-linked consensus sequencing enables phylogenetic analysis of directed evolution. Nat. Commun. 11, 6023 (2020).
Wilson, B. D., Eisenstein, M. & Soh, H. T. High-fidelity nanopore sequencing of ultra-short DNA targets. Anal. Chem. 91, 6783–6789 (2019).
Murray, K. D. & Borevitz, J. O. Axe: rapid, competitive sequence read demultiplexing using a trie. Bioinformatics 34, 3924–3925 (2018).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018).
Koboldt, D. C. et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 22, 568–576 (2012).
Deatherage, D. E. & Barrick, J. E. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods. Mol. Biol. 1151, 165–188 (2014).
Fowler, D. M. & Fields, S. Deep mutational scanning: a new style of protein science. Nat. Methods. 11, 801–807 (2014).
Sirawaraporn, W., Sathitkul, T., Sirawaraporn, R., Yuthavong, Y. & Santi, D. V. Antifolate-resistant mutants of Plasmodium falciparum dihydrofolate reductase. Biochemistry 94, 1124–1129 (1997).
Hankins, E. G., Warhurst, D. C. & Sibley, C. H. Novel alleles of the Plasmodium falciparum dhfr highly resistant to pyrimethamine and chlorcycloguanil, but not WR99210. Mol. Biochem. Parasitol. 117, 91–102 (2001).
Long, M. et al. Directed evolution of ornithine cyclodeaminase using an EvolvR-based growth-coupling strategy for efficient biosynthesis of l-proline. ACS Synth. Biol. 9, 1855–1863 (2020).
García-García, J. D. et al. Potential for applying continuous directed evolution to plant enzymes: an exploratory study. Life 10, 1–16 (2020).
García-García, J. D. et al. Using continuous directed evolution to improve enzymes for plant applications. Plant. Physiol. 188, 971–983 (2022).
Chatterjee, A. et al. Saccharomyces cerevisiae THI4p is a suicide thiamine thiazole synthase. Nature 478, 542–546 (2011).
Joshi, J. et al. Structure and function of aerotolerant, multiple-turnover THI4 thiazole synthases. Biochem. J. 478, 3265–3279 (2021).
Rix, G. et al. Scalable continuous evolution for the generation of diverse enzyme variants encompassing promiscuous activities. Nat. Commun. 11, 5644 (2020). This work demonstrates the use of OrthoRep to evolve in a scalable manner a large collection of highly diverse orthologues of an enzyme (TrpB), which was mined for promiscuous activities leading to the biosynthesis of valuable chemicals.
Dunn, M. F. Allosteric regulation of substrate channeling and catalysis in the tryptophan synthase bienzyme complex. Arch. Biochem. Biophys. 519, 154–166 (2012).
Buller, A. R. et al. Directed evolution of the tryptophan synthase β-subunit for stand-alone function recapitulates allosteric activation. Proc. Natl Acad. Sci. USA 112, 14599–14604 (2015).
Watkins-Dulaney, E., Straathof, S. & Arnold, F. Tryptophan synthase: biocatalyst extraordinaire. ChemBioChem 22, 5–16 (2021).
Romney, D. K., Murciano-Calles, J., Wehrmüller, J. E. & Arnold, F. H. Unlocking reactivity of TrpB: a general biocatalytic platform for synthesis of tryptophan analogues. J. Am. Chem. Soc. 139, 10769–10776 (2017).
Boville, C. E., Romney, D. K., Almhjell, P. J., Sieben, M. & Arnold, F. H. Improved synthesis of 4-cyanotryptophan and other tryptophan analogues in aqueous solvent using variants of TrpB from Thermotoga maritima. J. Org. Chem. 83, 7447–7452 (2018).
Javanpour, A. A. & Liu, C. C. Evolving small-molecule biosensors with improved performance and reprogrammed ligand preference using OrthoRep. ACS Synth. Biol. 10, 2705–2714 (2021).
Jensen, E. D. et al. Integrating continuous hypermutation with high-throughput screening for optimization of cis,cis-muconic acid production in yeast. Microb. Biotechnol. 14, 2617–2626 (2021).
Wellner, A. et al. Rapid generation of potent antibodies by autonomous hypermutation in yeast. Nat. Chem. Biol. 17, 1057–1064 (2021). This work demonstrates the use of OrthoRep to drive the rapid evolution of custom antibodies displayed on the surface of yeast cells, including nanomolar-affinity nanobodies that bind and neutralize SARS-CoV-2.
Pezo, V. et al. Noncanonical DNA polymerization by aminoadenine-based siphoviruses. Science 372, 520–524 (2021).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
Ling, X. et al. Improving the efficiency of precise genome editing with site-specific Cas9–oligonucleotide conjugates. Sci. Adv. 6, 1–9 (2020).
Wang, C. et al. Microbial single-strand annealing proteins enable CRISPR gene-editing tools with improved knock-in efficiencies and reduced off-target effects. Nucleic Acids Res. 49, 1–16 (2021).
Stevens, A. J. et al. Design of a split intein with exceptional protein splicing activity. J. Am. Chem. Soc. 138, 2162–2165 (2016).
Mills, D. R., Peterson, R. L. & Spiegelman, S. An extracellular Darwinian experiment with a self-duplicating nucleic acid molecule. Proc. Natl Acad. Sci. USA 58, 217–224 (1967).
Beaudry, A. A. & Joyce, G. F. Directed evolution of an RNA enzyme. Science 257, 635–641 (1992).
Leman, J. K. et al. Macromolecular modeling and design in Rosetta: recent methods and frameworks. Nat. Methods. 17, 665–680 (2020).
Stoltenburg, R., Reinemann, C. & Strehlitz, B. SELEX — a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 24, 381–403 (2007).
Torrisi, M., Pollastri, G. & Le, Q. Deep learning methods in protein structure prediction. Comput. Struct. Biotechnol. J. 18, 1301–1310 (2020).
Rollins, N. J. et al. Inferring protein 3D structure from deep mutation scans. Nat. Genet. 51, 1170–1176 (2019).
Langan, R. A. et al. De novo design of bioactive protein switches. Nature 572, 205–210 (2019).
Dou, J. et al. De novo design of a fluorescence-activating β-barrel. Nature 561, 485–491 (2018).
Basanta, B. et al. An enumerative algorithm for de novo design of proteins with diverse pocket structures. Proc. Natl Acad. Sci. USA 117, 22135–22145 (2020).
Anishchenko, I. et al. De novo protein design by deep network hallucination. Nature 600, 547–552 (2021).
Hadadi, N., MohammadiPeyhani, H., Miskovic, L., Seijo, M. & Hatzimanikatis, V. Enzyme annotation for orphan and novel reactions using knowledge of substrate reactive sites. Proc. Natl Acad. Sci. USA 116, 7298–7307 (2019).
Gumulya, Y. et al. Engineering highly functional thermostable proteins using ancestral sequence reconstruction. Nat. Catal. 1, 878–888 (2018).
Xie, V. C., Pu, J., Metzger, B. P. H., Thornton, J. W. & Dickinson, B. C. Contingency and chance erase necessity in the experimental evolution of ancestral proteins. eLife 10, 1–87 (2021).
Kaltenbach, M. et al. Evolution of chalcone isomerase from a noncatalytic ancestor article. Nat. Chem. Biol. 14, 548–555 (2018).
Biebricher, C. K. & Eigen, M. The error threshold. Virus Res. 107, 117–127 (2005).
Pu, J., Zinkus-Boltz, J. & Dickinson, B. C. Evolution of a split RNA polymerase as a versatile biosensor platform. Nat. Chem. Biol. 13, 432–438 (2017).
Packer, M. S., Rees, H. A. & Liu, D. R. Phage-assisted continuous evolution of proteases with altered substrate specificity. Nat. Commun. 8, 956 (2017).
Badran, A. H. et al. Continuous evolution of Bacillus thuringiensis toxins overcomes insect resistance. Nature 533, 58–63 (2016).
Inamoto, I., Sheoran, I., Popa, S. C., Hussain, M. & Shin, J. A. Combining rational design and continuous evolution on minimalist proteins that target the E-box DNA site. ACS Chem. Biol. 16, 35–44 (2021).
Wang, T., Badran, A. H., Huang, T. P. & Liu, D. R. Continuous directed evolution of proteins with improved soluble expression. Nat. Chem. Biol. 14, 972–980 (2018).
Thuronyi, B. W. et al. Continuous evolution of base editors with expanded target compatibility and improved activity. Nat. Biotechnol. 37, 1070–1079 (2019).
Berman, C. M. et al. An adaptable platform for directed evolution in human cells. J. Am. Chem. Soc. 140, 18093–18103 (2018).
English, J. G. et al. VEGAS as a platform for facile directed evolution in mammalian cells. Cell 178, 748–761.e17 (2019).
Acknowledgements
The authors thank members of their groups for insightful discussions. This work was funded by National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) 1R35GM139513 (C.C.L.); NIH NIGMS 1R35GM136354 (M.D.S.); MIT Robert J Silbey Fellowship (A.A.M.); MIT School of Science Fund for Future of Science (A.A.M.); US Department of Energy, Office of Science, Basic Energy Sciences under Award DE-SC0020153 (A.D.H.); Innovative Genomics Institute and Laboratory for Genomics Research (J.E.H., J.E.D. and D.V.S.); UC Berkeley Miller Basic Research Fellowship (Q.Z.); NIH National Institute of Biomedical Imaging and Bioengineering (NIBIB) 1R01EB027793 (A.S.K.); Department of Defense (DoD) Vannevar Bush Faculty Fellowship N00014-20-1-2825 (A.S.K.); NIH NIGMS 1R01GM125887 (F.H.A.); and Ministerio de Ciencia e Innovación - Consejo Superior de Investigaciones Científicas (MICIN-CSIC) PTI + REC-EU SGL2103051 and EU Horizon 2020 research and innovation programme FET Open 965018-BIOCELLPHE (L.A.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding agencies.
Author information
Authors and Affiliations
Contributions
Introduction (R.S.M., G.R., A.A.M., M.D.S. and C.C.L.); Experimentation (R.S.M., G.R., A.A.M., B.A., D.S., H.C., J.E.H., Q.Z., J.E.D., D.V.S., F.C., S.K., L.A.F., M.D.S. and C.C.L.); Results (R.S.M., G.R. and C.C.L.); Applications (R.S.M., G.R., A.A.M., B.A., D.S., H.C., J.E.H., Q.Z., J.D.G.-G., Z.J.H., P.J.A., F.H.A., A.S.K., A.D.H., J.E.D., D.V.S., F.C., S.K., L.A.F., M.D.S. and C.C.L.); Reproducibility and data deposition (R.S.M., G.R. and C.C.L.); Limitations and optimizations (R.S.M., G.R., A.A.M., B.A., D.S., H.C., J.E.H., Q.Z., J.E.D., D.V.S., F.C., S.K., L.A.F., M.D.S. and C.C.L.); Outlook (R.S.M., G.R. and C.C.L.). R.S.M. and G.R. contributed equally and are listed in alphabetical order.
Corresponding author
Ethics declarations
Competing interests
C.C.L. is a co-founder of K2 Biotechnologies, which applies continuous evolution to antibody engineering. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Jumi Shin, Chong Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
European Nucleotide Archive (ENA): https://www.ebi.ac.uk/ena/browser/home
National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA): https://www.ncbi.nlm.nih.gov/sra
NCBI BioProject: https://www.ncbi.nlm.nih.gov/bioproject/
Supplementary information
Glossary
- Directed evolution
-
A method that employs the evolutionary process of mutation, amplification and screening or selection to improve a protein or other biomolecule towards a desired function on laboratory timescales.
- Hypermutation
-
A marked increase in the mutation rate of a DNA sequence.
- Clonal interference
-
When one clone with a (new) beneficial mutation fails to fix because another lineage with a (new) beneficial mutation arises in the same population, common in asexual populations when mutation rates are high.
- Integration cassettes
-
Pieces of DNA designed to integrate into a specific location within another piece of DNA such as a genome or a plasmid.
- Processivity
-
The ability of an enzyme to catalyse multiple consecutive reactions without releasing its substrate.
- Cheater mutations
-
Mutations that allow a cell to satisfy selection without actually improving the desired function of the biomolecule under evolution.
- Unique molecular identifier
-
(UMI). A random barcode added to sequencing libraries to differentiate individual molecules from each other before amplification.
- Consensus sequencing
-
An approach used in high-throughput sequencing (HTS) that corrects errors by sequencing a particular sequence multiple times and taking the consensus.
- Greedy mutations
-
Single mutations representing the locally optimal choice for improving the function of a gene during a given stage of evolution.
- Sign epistasis
-
When one mutation that has a particular effect on the desired biomolecular function causes the opposite effect when it is in the presence of another mutation.
Rights and permissions
About this article
Cite this article
Molina, R.S., Rix, G., Mengiste, A.A. et al. In vivo hypermutation and continuous evolution. Nat Rev Methods Primers 2, 36 (2022). https://doi.org/10.1038/s43586-022-00119-5
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43586-022-00119-5
This article is cited by
-
Directed evolution of functional intrinsically disordered proteins
Nature Chemical Biology (2026)
-
Fueling chromosomal gene diversification and artificial evolution with CRISPR
Genome Biology (2025)
-
Directed evolution of hydrocarbon-producing enzymes
Biotechnology for Biofuels and Bioproducts (2025)
-
Tuning evolvability via plasmid copy number and regulatory architecture
Nature Communications (2025)
-
The role of plasmid copy number and mutation rate in evolutionary outcomes
Nature Ecology & Evolution (2025)