Abstract
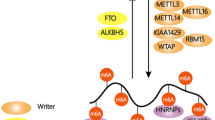
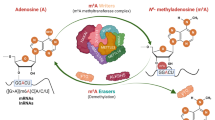
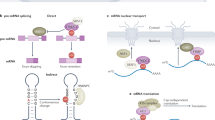
N6-methyladenosine (m6A) is the most prevalent internal mRNA modification. Recent research has highlighted its role as a key regulator of gene expression, influencing cellular processes and determining cell fate. Advances in techniques for global mapping of m6A, the discovery of m6A demethylases that enhance its dynamic properties and the identification of reader proteins that interact with m6A have substantially propelled this field forward. This Primer outlines the available tools for detecting and mapping m6A, discusses the strengths and limitations of each method and offers guidance on selecting the most suitable approach. Identifying and detecting m6A lays the groundwork for functional studies that address important biological and medical questions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Desrosiers, R., Friderici, K. & Rottman, F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl Acad. Sci. USA 71, 3971–3975 (1974).
Dominissini, D. et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206 (2012).
Meyer, K. D. et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646 (2012).
Jia, G. et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 7, 885–887 (2011).
Zheng, G. et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29 (2013).
Dominissini, D. et al. The dynamic N1-methyladenosine methylome in eukaryotic messenger RNA. Nature 530, 441–446 (2016).
Levanon, E. Y. et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat. Biotechnol. 22, 1001–1005 (2004).
Squires, J. E. et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 40, 5023–5033 (2012).
Delatte, B. et al. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science 351, 282–285 (2016).
Carlile, T. M., Rojas-Duran, M. F. & Gilbert, W. V. in Methods in Enzymology Vol. 560, 219–245 (Elsevier, 2015).
Li, X. et al. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat. Chem. Biol. 11, 592–597 (2015).
Schwartz, S. et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 159, 148–162 (2014).
Akichika, S. et al. Cap-specific terminal N6-methylation of RNA by an RNA polymerase II-associated methyltransferase. Science 363, eaav0080 (2019).
Mauer, J. et al. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature 541, 371–375 (2017).
Sun, H., Zhang, M., Li, K., Bai, D. & Yi, C. Cap-specific, terminal N6-methylation by a mammalian m6Am methyltransferase. Cell Res. 29, 80–82 (2019).
Arango, D. et al. Acetylation of cytidine in mRNA promotes translation efficiency. Cell 175, 1872–1886.e24 (2018).
Zhang, L.-S. et al. Transcriptome-wide mapping of internal N7-methylguanosine methylome in mammalian mRNA. Mol. Cell 74, 1304–1316.e8 (2019).
Dai, Q. et al. Nm-seq maps 2′-O-methylation sites in human mRNA with base precision. Nat. Methods 14, 695–698 (2017).
Liu, J. E. et al. Landscape and regulation of m6A and m6Am methylome across human and mouse tissues. Mol. Cell 77, 426–440.e6 (2020).
Liu, N. et al. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA 19, 1848–1856 (2013).
Wei, J. et al. Differential m6A, m6Am, and m1A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol. Cell 71, 973–985.e5 (2018).
Sendinc, E. et al. PCIF1 catalyzes m6Am mRNA methylation to regulate gene expression. Mol. Cell 75, 620–630.e9 (2019).
Hu, J., Xu, T. & Kang, H. Crosstalk between RNA m6A modification and epigenetic factors in plant gene regulation. Plant Commun. 5, 101037 (2024).
Garcias Morales, D. & Reyes, J. L. A birds’-eye view of the activity and specificity of the mRNA mA methyltransferase complex. WIREs RNA 12, e1618 (2021).
Zhang, W., Qian, Y. & Jia, G. The detection and functions of RNA modification m6A based on m6A writers and erasers. J. Biol. Chem. 297, 100973 (2021).
Knuckles, P. et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m6A machinery component Wtap/Fl(2)d. Genes Dev. 32, 415–429 (2018).
Ensinck, I. et al. The yeast RNA methylation complex consists of conserved yet reconfigured components with m6A-dependent and independent roles. eLife 12, RP87860 (2023).
Mermoud, J. E. The role of the m6A RNA methyltransferase METTL16 in gene expression and SAM homeostasis. Genes 13, 2312 (2022).
Satterwhite, E. R. & Mansfield, K. D. RNA methyltransferase METTL16: targets and function. WIREs RNA 13, e1681 (2022).
Lence, T., Paolantoni, C., Worpenberg, L. & Roignant, J.-Y. Mechanistic insights into m6A RNA enzymes. Biochim. Biophys. Acta Gene Regul. Mech. 1862, 222–229 (2019).
Garcia-Campos, M. A. et al. Deciphering the ‘m6A code’ via antibody-independent quantitative profiling. Cell 178, 731–747.e16 (2019).
Gerken, T. et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318, 1469–1472 (2007).
Herr, C. Q. & Hausinger, R. P. Amazing diversity in biochemical roles of Fe (II)/2-oxoglutarate oxygenases. Trends Biochem. Sci. 43, 517–532 (2018).
Liao, S., Sun, H. & Xu, C. YTH domain: a family of N(6)-methyladenosine (m(6)A) readers. Genomics Proteom. Bioinforma. 16, 99–107 (2018).
Duan, M. et al. IGF2BPs as novel m(6)A readers: diverse roles in regulating cancer cell biological functions, hypoxia adaptation, metabolism, and immunosuppressive tumor microenvironment. Genes Dis. 11, 890–920 (2024).
Liu, N. et al. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 45, 6051–6063 (2017).
Alarcón & Claudio, R. et al. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events. Cell 162, 1299–1308 (2015).
Liu, N. et al. N6-methyladenosine-dependent RNA structural switches regulate RNA–protein interactions. Nature 518, 560–564 (2015).
Arguello, A. E., DeLiberto, A. N. & Kleiner, R. E. RNA chemical proteomics reveals the N6-methyladenosine (m6A)-regulated protein–RNA interactome. J. Am. Chem. Soc. 139, 17249–17252 (2017).
Edupuganti, R. R. et al. N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat. Struct. Mol. Biol. 24, 870–878 (2017).
Dominissini, D. & Rechavi, G. Epitranscriptome regulation [poster]. Nat. Struct. Mol. Biol. https://doi.org/10.1038/s41594-018-0140-7 (2018).
Xiao, W. et al. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol. Cell 61, 507–519 (2016).
Kasowitz, S. D. et al. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet. 14, e1007412 (2018).
Chen, L. et al. Nuclear m6A reader YTHDC1 suppresses proximal alternative polyadenylation sites by interfering with the 3′ processing machinery. EMBO Rep. 23, e54686 (2022).
Roundtree, I. A. et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. eLife 6, e31311 (2017).
Wang, X. et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120 (2014).
Cheng, Y. et al. N6-methyladenosine on mRNA facilitates a phase-separated nuclear body that suppresses myeloid leukemic differentiation. Cancer Cell 39, 958–972.e8 (2021).
Du, H. et al. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4–NOT deadenylase complex. Nat. Commun. 7, 12626 (2016).
Huang, H. et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 20, 285–295 (2018).
Geula, S. et al. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 347, 1002–1006 (2015).
Zhang, Q., Dong, L., Gong, S. & Wang, T. Unraveling the landscape of m6A RNA methylation in wound healing and scars. Cell Death Discov. 10, 458 (2024).
Zhang, Y., Hu, W. & Li, H.-B. RNA modification-mediated translational control in immune cells. RNA Biol. 20, 603–613 (2023).
Zhao, M. et al. N6-methyladenosine modification of TSC1 mRNA contributes to macrophage polarization regulated by Coptisine in DSS-induced ulcerative colitis. Phytomedicine 122, 155153 (2024).
Shen, L., Liang, Z. & Yu, H. Dot blot analysis of N6-methyladenosine RNA modification levels. bio-protocol 7, e2095 (2017).
Desrosiers, R. C., Friderici, K. H. & Rottman, F. M. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5′-terminus. Biochemistry 14, 4367–4374 (1975).
Gehrke, C. W. & Kuo, K. C. Ribonucleoside analysis by reversed-phase high-performance liquid chromatography. J. Chromatogr. 471, 3–36 (1989).
Buck, M., Connick, M. & Ames, B. N. Complete analysis of tRNA-modified nucleosides by high-performance liquid chromatography: the 29 modified nucleosides of Salmonella typhimurium and Escherichia coli tRNA. Anal. Biochem. 129, 1–13 (1983).
Grosjean, H., Droogmans, L., Roovers, M. & Keith, G. in Methods in Enzymology Vol. 425, 55–101 (Academic Press, 2007).
Ensinck, I. et al. m6A-ELISA, a simple method for quantifying N6-methyladenosine from mRNA populations. RNA 29, 705–712 (2023).
Lauman, R. & Garcia, B. A. Unraveling the RNA modification code with mass spectrometry. Mol. Omics 16, 305–315 (2020).
Su, D. et al. Quantitative analysis of ribonucleoside modifications in tRNA by HPLC-coupled mass spectrometry. Nat. Protoc. 9, 828–841 (2014).
Sarin, L. P. et al. Nano LC–MS using capillary columns enables accurate quantification of modified ribonucleosides at low femtomol levels. RNA 24, 1403–1417 (2018).
Basanta-Sanchez, M., Temple, S., Ansari, S. A., D’Amico, A. & Agris, P.F. Attomole quantification and global profile of RNA modifications: Epitranscriptome of human neural stem cells. Nucleic Acids Res. 44, e26 (2016).
Li, S. & Limbach, P. A. Method for comparative analysis of ribonucleic acids using isotope labeling and mass spectrometry. Anal. Chem. 84, 8607–8613 (2012).
Paulines, M. J. & Limbach, P. A. Stable isotope labeling for improved comparative analysis of RNA digests by mass spectrometry. J. Am. Soc. Mass. Spectrom. 28, 551–561 (2017).
Sun, H. et al. m6Am-seq reveals the dynamic m6Am methylation in the human transcriptome. Nat. Commun. 12, 4778 (2021).
Solivio, B., Yu, N., Addepalli, B. & Limbach, P. A. Improving RNA modification mapping sequence coverage by LC–MS through a nonspecific RNase U2-E49A mutant. Anal. Chim. Acta 1036, 73–79 (2018).
Mirza, A. H., Attarwala, N., Gross, S. S., Chen, Q. & Jaffrey, S. R. Selective detection of m6A derived from mRNA using the Phospho-tag m6A assay. Preprint at bioRxiv https://doi.org/10.1101/2022.05.23.493172 (2022).
Dominissini, D., Moshitch-Moshkovitz, S., Salmon-Divon, M., Amariglio, N. & Rechavi, G. Transcriptome-wide mapping of N6-methyladenosine by m6A-seq based on immunocapturing and massively parallel sequencing. Nat. Protoc. 8, 176–189 (2013).
Dierks, D. et al. Multiplexed profiling facilitates robust m6A quantification at site, gene and sample resolution. Nat. Methods 18, 1060–1067 (2021).
Linder, B. et al. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat. Methods 12, 767–772 (2015).
Licatalosi, D. D. et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature 456, 464–469 (2008).
Darnell, R. B. HITS-CLIP: panoramic views of protein–RNA regulation in living cells. WIREs RNA 1, 266–286 (2010).
Dziuba, D., Hoffmann, J.-E., Hentze, M. W. & Schultz, C. A genetically encoded diazirine analogue for RNA–protein photo-crosslinking. ChemBioChem 21, 88–93 (2020).
Ke, S. et al. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 29, 2037–2053 (2015).
Körtel, N. et al. Deep and accurate detection of m6A RNA modifications using miCLIP2 and m6Aboost machine learning. Nucleic Acids Res. 49, e92 (2021).
Chen, K. et al. High-resolution N(6)-methyladenosine (m(6)A) map using photo-crosslinking-assisted m(6)A sequencing. Angew. Chem. Int. Ed. 54, 1587–1590 (2015).
Koh, C. W. Q., Goh, Y. T. & Goh, W. S. S. Atlas of quantitative single-base-resolution N6-methyl-adenine methylomes. Nat. Commun. 10, 5636 (2019).
Molinie, B. et al. m6A-LAIC-seq reveals the census and complexity of the m6A epitranscriptome. Nat. Methods 13, 692–698 (2016).
Yin, R. et al. Differential m6A RNA landscapes across hematopoiesis reveal a role for IGF2BP2 in preserving hematopoietic stem cell function. Cell Stem Cell 29, 149–159.e7 (2022).
Ramsköld, D. et al. Full-length mRNA-seq from single-cell levels of RNA and individual circulating tumor cells. Nat. Biotechnol. 30, 777–782 (2012).
Li, Y. et al. Single-cell m6A mapping in vivo using picoMeRIP-seq. Nat. Biotechnol. 42, 591–596 (2023).
Yao, H. et al. scm6A-seq reveals single-cell landscapes of the dynamic m6A during oocyte maturation and early embryonic development. Nat. Commun. 14, 315 (2023).
Grozhik, A. V. et al. Antibody cross-reactivity accounts for widespread appearance of m1A in 5′UTRs. Nat. Commun. 10, 5126 (2019).
Moshitch-Moshkovitz, S., Heldman, Y., Yayon, A. & Katchalski-Katzir, E. Sorting polyclonal antibodies into functionally distinct fractions using peptide phage display: ‘a library on top of a library’. J. Immunol. Methods 242, 183–191 (2000).
Moshitch-Moshkovitz, S., Dominissini, D. & Rechavi, G. The epitranscriptome toolbox. Cell 185, 764–776 (2022).
Shu, X., Cao, J. & Liu, J. m6A-label-seq: a metabolic labeling protocol to detect transcriptome-wide mRNA N6-methyladenosine (m6A) at base resolution. STAR Protoc. 3, 101096 (2022).
Shu, X. et al. A metabolic labeling method detects m6A transcriptome-wide at single base resolution. Nat. Chem. Biol. 16, 887–895 (2020).
Meyer, K. D. DART-seq: an antibody-free method for global m6A detection. Nat. Methods 16, 1275–1280 (2019).
Xu, C. et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat. Chem. Biol. 10, 927–929 (2014).
Shen, D. et al. Detailed resume of RNA m6A demethylases. Acta Pharm. Sin. B 12, 2193–2205 (2022).
Blanc, V. & Davidson, N. O. APOBEC‐1‐mediated RNA editing. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 594–602 (2010).
Tegowski, M., Flamand, M. N. & Meyer, K. D. scDART-seq reveals distinct m6A signatures and mRNA methylation heterogeneity in single cells. Mol. Cell 82, 868–878.e10 (2022).
Wang, Y., Xiao, Y., Dong, S., Yu, Q. & Jia, G. Antibody-free enzyme-assisted chemical approach for detection of N6-methyladenosine. Nat. Chem. Biol. 16, 896–903 (2020).
Ge, R. et al. m6A-SAC-seq for quantitative whole transcriptome m6A profiling. Nat. Protoc. 18, 626–657 (2023).
Chen, H.-X., Zhang, Z., Ma, D.-Z., Chen, L.-Q. & Luo, G.-Z. Mapping single-nucleotide m6A by m6A-REF-seq. Methods 203, 392–398 (2022).
Zhang, Z. et al. Systematic calibration of epitranscriptomic maps using a synthetic modification-free RNA library. Nat. Methods 18, 1213–1222 (2021).
Keegan, L. P., Leroy, A., Sproul, D. & O’Connell, M. A. Adenosine deaminases acting on RNA (ADARs): RNA-editing enzymes. Genome Biol. 5, 209 (2004).
Xiang, J. F. et al. N(6)-methyladenosines modulate A-to-I RNA editing. Mol. Cell 69, 126–135.e6 (2018).
Véliz, E. A., Easterwood, L. M. & Beal, P. A. Substrate analogues for an RNA-editing adenosine deaminase: mechanistic investigation and inhibitor design. J. Am. Chem. Soc. 125, 10867–10876 (2003).
Xiao, Y.-L. et al. Transcriptome-wide profiling and quantification of N6-methyladenosine by enzyme-assisted adenosine deamination. Nat. Biotechnol. 41, 993–1003 (2023).
Shen, W. et al. GLORI for absolute quantification of transcriptome-wide m6A at single-base resolution. Nat. Protoc. 19, 1252–1287 (2024).
Liu, C. et al. Absolute quantification of single-base m6A methylation in the mammalian transcriptome using GLORI. Nat. Biotechnol. 41, 355–366 (2023).
Xiao, Y. et al. An elongation- and ligation-based qPCR amplification method for the radiolabeling-free detection of locus-specific N6-methyladenosine modification. Angew. Chem. Int. Ed. 57, 15995–16000 (2018).
Liu, W. et al. Identification of a selective DNA ligase for accurate recognition and ultrasensitive quantification of N6-methyladenosine in RNA at one-nucleotide resolution. Chem. Sci. 9, 3354–3359 (2018).
Garalde, D. R. et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat. Methods 15, 201–206 (2018).
Workman, R. E. et al. Nanopore native RNA sequencing of a human poly (A) transcriptome. Nat. Methods 16, 1297–1305 (2019).
Wang, Y., Zhao, Y., Bollas, A., Wang, Y. & Au, K. F. Nanopore sequencing technology, bioinformatics and applications. Nat. Biotechnol. 39, 1348–1365 (2021).
Furlan, M. et al. Computational methods for RNA modification detection from nanopore direct RNA sequencing data. RNA Biol. 18, 31–40 (2021).
Price, A. M. et al. Direct RNA sequencing reveals m(6)A modifications on adenovirus RNA are necessary for efficient splicing. Nat. Commun. 11, 6016 (2020).
Leger, A. et al. RNA modifications detection by comparative nanopore direct RNA sequencing. Nat. Commun. 12, 7198 (2021).
Pratanwanich, P. N. et al. Identification of differential RNA modifications from nanopore direct RNA sequencing with xPore. Nat. Biotechnol. 39, 1394–1402 (2021).
Liu, H., Begik, O. & Novoa, E. M. in RNA Modifications: Methods and Protocols (ed. McMahon, M.) 31–52 (Springer US, 2021).
Lorenz, D. A., Sathe, S., Einstein, J. M. & Yeo, G. W. Direct RNA sequencing enables m(6)A detection in endogenous transcript isoforms at base-specific resolution. RNA 26, 19–28 (2020).
Gao, Y. et al. Quantitative profiling of N(6)-methyladenosine at single-base resolution in stem-differentiating xylem of Populus trichocarpa using Nanopore direct RNA sequencing. Genome Biol. 22, 22 (2021).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2012).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Smith, T., Heger, A. & Sudbery, I. UMI-tools: modeling sequencing errors in unique molecular identifiers to improve quantification accuracy. Genome Res. 27, 491–499 (2017).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Meng, J., Cui, X., Rao, M. K., Chen, Y. & Huang, Y. Exome-based analysis for RNA epigenome sequencing data. Bioinformatics 29, 1565–1567 (2013).
Cui, X. et al. MeTDiff: a novel differential RNA methylation analysis for MeRIP-seq data. IEEE/ACM Trans. Comput. Biol. Bioinform. 15, 526–534 (2018).
Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27, 2987–2993 (2011).
Yao, Z. et al. Evaluation of variant calling tools for large plant genome re-sequencing. BMC Bioinformatics 21, 1–16 (2020).
Danecek, P. & McCarthy, S. A. BCFtools/csq: haplotype-aware variant consequences. Bioinformatics 33, 2037–2039 (2017).
Poh, H. X. Mirza, A. H., Pickering, B. F. & Jaffrey, S. R. Alternative splicing of METTL3 explains apparently METTL3-independent m6A modifications in mRNA. PLoS Biol. 20, e3001683 (2022).
Schwartz, S. et al. High-resolution mapping reveals a conserved widespread dynamic mRNA methylation program in yeast meiosis. Cell 155, 1409–1421 (2013).
Varier, R. A. et al. N6-methyladenosine (m6A) reader Pho92 is recruited co-transcriptionally and couples translation to mRNA decay to promote meiotic fitness in yeast. eLife 11, e84034 (2022).
Zhou, J. et al. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594 (2015).
Dominissini, D. & Rechavi, G. Loud and clear epitranscriptomic m1A signals: now in single-base resolution. Mol. Cell 68, 825–826 (2017).
Batista, P. J. et al. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 15, 707–719 (2014).
Wang, Y. et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 16, 191–198 (2014).
Lin, Z. & Tong, M.-H. m6A mRNA modification regulates mammalian spermatogenesis. Biochim. Biophys. Acta Gene Regul. Mech. 1862, 403–411 (2019).
Tan, X., Zheng, C., Zhuang, Y., Jin, P. & Wang, F. The m6A reader PRRC2A is essential for meiosis I completion during spermatogenesis. Nat. Commun. 14, 1636 (2023).
Qi, M. et al. m6A reader protein YTHDF2 regulates spermatogenesis by timely clearance of phase‐specific transcripts. Cell Prolif. 55, e13164 (2022).
Gu, Y., Song, Y., Pan, Y. & Liu, J. The essential roles of m6A modification in osteogenesis and common bone diseases. Genes Dis. 11, 335–345 (2023).
He, M. et al. METTL14 regulates osteogenesis of bone marrow mesenchymal stem cells via inducing autophagy through m6A/IGF2BPs/Beclin-1 signal axis. Stem Cell Transl. Med. 11, 987–1001 (2022).
Liu, T. et al. The m6A ‘reader’ YTHDF1 promotes osteogenesis of bone marrow mesenchymal stem cells through translational control of ZNF839. Cell Death Dis. 12, 1078 (2021).
Zhao, X. et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 24, 1403–1419 (2014).
Wang, X. et al. m(6)A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy 16, 1221–1235 (2020).
Yang, Z., Yu, G. L., Zhu, X., Peng, T. H. & Lv, Y. C. Critical roles of FTO-mediated mRNA m6A demethylation in regulating adipogenesis and lipid metabolism: implications in lipid metabolic disorders. Genes Dis. 9, 51–61 (2022).
Yen, Y. P. & Chen, J. A. The m(6)A epitranscriptome on neural development and degeneration. J. Biomed. Sci. 28, 40 (2021).
Yoon, K. J. et al. Temporal control of mammalian cortical neurogenesis by m(6)A methylation. Cell 171, 877–889.e17 (2017).
Livneh, I., Moshitch-Moshkovitz, S., Amariglio, N., Rechavi, G. & Dominissini, D. The m(6)A epitranscriptome: transcriptome plasticity in brain development and function. Nat. Rev. Neurosci. 21, 36–51 (2020).
Sun, T., Wu, R. & Ming, L. The role of m6A RNA methylation in cancer. Biomed. Pharmacother. 112, 108613 (2019).
Lan, Q. et al. The critical role of RNA m6A methylation in cancer. Cancer Res. 79, 1285–1292 (2019).
Huang, H., Weng, H. & Chen, J. m6A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell 37, 270–288 (2020).
Liu, H.-T. et al. Deciphering the divergent gene expression landscapes of m6A/m5C/m1A methylation regulators in hepatocellular carcinoma through single-cell and bulk RNA transcriptomic analysis. J. Hepatocell. Carcinoma 10, 2383–2395 (2023).
Yuan, F. et al. Roles of the m6A modification of RNA in the glioblastoma microenvironment as revealed by single-cell analyses. Front. Immunol. 13, 798583 (2022).
Li, Z. et al. Integrated analysis of single-cell RNA-seq and bulk RNA-seq reveals RNA N6-methyladenosine modification associated with prognosis and drug resistance in acute myeloid leukemia. Front. Immunol. 14, 1281687 (2023).
Gao, Y. et al. Single-cell N6-methyladenosine regulator patterns guide intercellular communication of tumor microenvironment that contribute to colorectal cancer progression and immunotherapy. J. Transl. Med. 20, 197 (2022).
Weng, H. et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m6A modification. Cell Stem Cell 22, 191–205.e9 (2018).
Vu, L. P. et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med. 23, 1369–1376 (2017).
Barbieri, I. et al. Promoter-bound METTL3 maintains myeloid leukaemia by m6A-dependent translation control. Nature 552, 126–131 (2017).
Li, Z. et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine RNA demethylase. Cancer Cell 31, 127–141 (2017).
Su, R. et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell 38, 79–96.e11 (2020).
Zhang, L. & Su, X. Bioactive peptide inhibits acute myeloid leukemia cell proliferation by downregulating ALKBH5-mediated m6A demethylation of EIF4EBP1 and MLST8 mRNA. Cell. Oncol. 45, 355–365 (2022).
Yan, F. et al. A dynamic N6-methyladenosine methylome regulates intrinsic and acquired resistance to tyrosine kinase inhibitors. Cell Res. 28, 1062–1076 (2018).
Hong, Y.-G. et al. The RNA m6A reader YTHDF1 is required for acute myeloid leukemia progression. Cancer Res. 83, 845–860 (2023).
Sheng, Y. et al. A critical role of nuclear m6A reader YTHDC1 in leukemogenesis by regulating MCM complex-mediated DNA replication. Blood 138, 2838–2852 (2021).
Hong, Y.-G. et al. The RNA m6A reader YTHDF1 promotes hematopoietic malignancy by maintaining oncogenic translation. Preprint at bioRxiv https://doi.org/10.1101/2022.10.08.511406 (2022).
Zhang, Z. et al. RNA m6A reader YTHDF2 facilitates precursor miR-126 maturation to promote acute myeloid leukemia progression. Genes Dis. 11, 382–396 (2024).
Zhang, L. et al. Targeting miR-126 in inv(16) acute myeloid leukemia inhibits leukemia development and leukemia stem cell maintenance. Nat. Commun. 12, 6154 (2021).
Caserta, C. et al. miR-126 identifies a quiescent and chemo-resistant human B-ALL cell subset that correlates with minimal residual disease. Leukemia 37, 1994–2005 (2023).
Wang, X. et al. Structural basis of N6-adenosine methylation by the METTL3–METTL14 complex. Nature 534, 575–578 (2016).
Fiorentino, F., Menna, M., Rotili, D., Valente, S. & Mai, A. METTL3 from target validation to the first small-molecule inhibitors: a medicinal chemistry journey. J. Med. Chem. 66, 1654–1677 (2023).
Bedi, R. K. et al. Small-molecule inhibitors of METTL3, the major human epitranscriptomic writer. ChemMedChem 15, 744–748 (2020).
Borchardt, R. T., Eiden, L. E., Wu, B. & Rutledge, C. O. Sinefungin, a potent inhibitor of S-adenosylmethionine: protein O-methyltransferase. Biochem. Biophys. Res. Commun. 89, 919–924 (1979).
Du, Y. et al. Discovery of METTL3 small molecule inhibitors by virtual screening of natural products. Front. Pharmacol. 13, 878135 (2022).
Li, H., Zhang, Q., Feng, Q., You, Q. & Guo, X. The development of small molecules targeting methyltransferase-like 3. Drug Discov. Today 28, 103513 (2023).
Yankova, E. et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature 593, 597–601 (2021).
Guirguis, A. A. et al. Inhibition of METTL3 results in a cell-intrinsic interferon response that enhances antitumor immunity. Cancer Discov. 13, 2228–2247 (2023).
Ofir-Rosenfeld, Y. et al. STC-15, an oral small molecule inhibitor of the RNA methyltransferase METTL3, inhibits tumour growth through activation of anti-cancer immune responses associated with increased interferon signalling, and synergises with T cell checkpoint blockade. Eur. J. Cancer 174, S123 (2022).
Li, Z. et al. A stapled peptide inhibitor targeting the binding interface of N6-adenosine-methyltransferase subunits METTL3 and METTL14 for cancer therapy. Angew. Chem. Int. Ed. 63, e202402611 (2024).
Dolbois, A. et al. 1,4,9-Triazaspiro[5.5]undecan-2-one derivatives as potent and selective METTL3 inhibitors. J. Med. Chem. 64, 12738–12760 (2021).
Moroz‐Omori, E. V. et al. METTL3 inhibitors for epitranscriptomic modulation of cellular processes. ChemMedChem 16, 3035–3043 (2021).
Chowdhury, R. et al. Selective small molecule probes for the hypoxia inducible factor (HIF) prolyl hydroxylases. ACS Chem. Biol. 8, 1488–1496 (2013).
Nakashima, Y., Brewitz, L., Tumber, A., Salah, E. & Schofield, C. J. 2-Oxoglutarate derivatives can selectively enhance or inhibit the activity of human oxygenases. Nat. Commun. 12, 6478 (2021).
Šimelis, K. et al. Selective targeting of human TET1 by cyclic peptide inhibitors: insights from biochemical profiling. Bioorg. Med. Chem. 99, 117597 (2024).
You, Y. et al. Recent advances of m6A demethylases inhibitors and their biological functions in human diseases. Int. J. Mol. Sci. 23, 5815 (2022).
Zhang, R., Li, Y., Wang, H., Zhu, K. & Zhang, G. Rhein regulates the proliferation and apoptosis of human leukaemia cells and its effects on the miR-27/CUL5 axis. Archiv. Med. Sci. https://doi.org/10.5114/aoms.2020.96711 (2020).
Canaani, J. et al. A phase II study of bisantrene in patients with relapsed/refractory acute myeloid leukemia. Eur. J. Haematol. 106, 260–266 (2021).
Boissel, S. et al. Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am. J. Hum. Genet. 85, 106–111 (2009).
Huang, T. et al. FTO knockout causes chromosome instability and G2/M arrest in mouse GC-1 cells. Front. Genet. 9, 732 (2019).
Carnevali, L. et al. Signs of cardiac autonomic imbalance and proarrhythmic remodeling in FTO deficient mice. PLoS ONE 9, e95499 (2014).
Merkestein, M. et al. FTO influences adipogenesis by regulating mitotic clonal expansion. Nat. Commun. 6, 6792 (2015).
Li, Y., Su, R., Deng, X., Chen, Y. & Chen, J. FTO in cancer: functions, molecular mechanisms, and therapeutic implications. Trends Cancer 8, 598–614 (2022).
Su, R. et al. Effective novel FTO inhibitors show potent anti-cancer efficacy and suppress drug resistance. Blood 134, 233 (2019).
Huang, Y. et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell 35, 677–691.e10 (2019).
Huang, Y. et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 43, 373–384 (2014).
Huff, S., Tiwari, S. K., Gonzalez, G. M., Wang, Y. & Rana, T. M. m(6)A-RNA demethylase FTO inhibitors impair self-renewal in glioblastoma stem cells. ACS Chem. Biol. 16, 324–333 (2021).
Sun, K. et al. Saikosaponin D exhibits anti-leukemic activity by targeting FTO/m(6)A signaling. Theranostics 11, 5831–5846 (2021).
Hu, R. et al. N6-methyladenosine RNA modifications: a potential therapeutic target for AML. Ann. Hematol. https://doi.org/10.1007/s00277-023-05302-6 (2023).
Papazoglou, P., Peng, L. & Sachinidis, A. Epigenetic mechanisms involved in the cardiovascular toxicity of anticancer drugs. Front. Cardiovasc. Med. 8, 658900 (2021).
Huang, Y., Xia, W., Dong, Z. & Yang, C.-G. Chemical inhibitors targeting the oncogenic m6A modifying proteins. Acc. Chem. Res. 56, 3010–3022 (2023).
Wang, Y.-Z. et al. Discovery of pyrazolo[1,5-a]pyrimidine derivative as a novel and selective ALKBH5 inhibitor for the treatment of AML. J. Med. Chem. 66, 15944–15959 (2023).
Lai, G.-Q. et al. A covalent compound selectively inhibits RNA demethylase ALKBH5 rather than FTO. RSC Chem. Biol. 5, 335–343 (2024).
Shi, P. & Wu, X. Programmable RNA targeting with CRISPR–Cas13. RNA Biol. 21, 1–9 (2024).
Wilson, C., Chen, P. J., Miao, Z. & Liu, D. R. Programmable m6A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat. Biotechnol. 38, 1431–1440 (2020).
Chang, C., Ma, G., Cheung, E. & Hutchins, A. P. A programmable system to methylate and demethylate N6-methyladenosine (m6A) on specific RNA transcripts in mammalian cells. J. Biol. Chem. 298, 102525 (2022).
Xuan, J.-J. et al. RMBase v2.0: deciphering the map of RNA modifications from epitranscriptome sequencing data. Nucleic Acids Res. 46, D327–D334 (2017).
Xuan, J. et al. RMBase v3.0: decode the landscape, mechanisms and functions of RNA modifications. Nucleic Acids Res. 52, D273–D284 (2023).
Yang, X. et al. 5-Methylcytosine promotes mRNA export — NSUN2 as the methyltransferase and ALYREF as an m5C reader. Cell Res. 27, 606–625 (2017).
Zhang, Y. et al. CDK13 promotes lipid deposition and prostate cancer progression by stimulating NSUN5-mediated m5C modification of ACC1 mRNA. Cell Death Differ. 30, 2462–2476 (2023).
Zheng, Q. et al. Cytoplasmic m1A reader YTHDF3 inhibits trophoblast invasion by downregulation of m1A-methylated IGF1R. Cell Discov. 6, 12 (2020).
Wu, Y. et al. RNA m(1)A methylation regulates glycolysis of cancer cells through modulating ATP5D. Proc. Natl Acad. Sci. USA 119, e2119038119 (2022).
Seo, K. W. & Kleiner, R. E. YTHDF2 recognition of N1-methyladenosine (m1A)-modified RNA is associated with transcript destabilization. ACS Chem. Biol. 15, 132–139 (2019).
Boo, S. H., Ha, H. & Kim, Y. K. m(1)A and m(6)A modifications function cooperatively to facilitate rapid mRNA degradation. Cell Rep. 40, 111317 (2022).
Biever, A., Donlin-Asp, P. G. & Schuman, E. M. Local translation in neuronal processes. Curr. Opin. Neurobiol. 57, 141–148 (2019).
Zeng, H. et al. Spatially resolved single-cell translatomics at molecular resolution. Science 380, eadd3067 (2023).
Deng, Y. et al. Spatial-CUT&tag: spatially resolved chromatin modification profiling at the cellular level. Science 375, 681–686 (2022).
Deng, Y. et al. Spatial profiling of chromatin accessibility in mouse and human tissues. Nature 609, 375–383 (2022).
Merkurjev, D. et al. Synaptic N6-methyladenosine (m6A) epitranscriptome reveals functional partitioning of localized transcripts. Nat. Neurosci. 21, 1004–1014 (2018).
Yu, J. et al. Dynamic m6A modification regulates local translation of mRNA in axons. Nucleic Acids Res. 46, 1412–1423 (2018).
Weng, Y.-L. et al. Epitranscriptomic m6A regulation of axon regeneration in the adult mammalian nervous system. Neuron 97, 313–325.e6 (2018).
Feng, Z. et al. Lighting up RNA-specific multi-photon and super-resolution imaging using a novel zinc complex. Nanoscale 15, 5486–5493 (2023).
Marayati, B. F., Thompson, M. G., Holley, C. L., Horner, S. M. & Meyer, K. D. Programmable protein expression using a genetically encoded m6A sensor. Nat. Biotechnol. 42, 1417–1428 (2024).
Selberg, S. et al. Discovery of small molecules that activate RNA methylation through cooperative binding to the METTL3-14-WTAP complex active site. Cell Rep. 26, 3762–3771.e5 (2019).
Li, J. & Gregory, R. I. Mining for METTL3 inhibitors to suppress cancer. Nat. Struct. Mol. Biol. 28, 460–462 (2021).
Sturgess, K. et al. Pharmacological inhibition of METTL3 impacts specific haematopoietic lineages. Leukemia 37, 2133–2137 (2023).
Brewer, G. METTL3 inhibition enhances anti-tumour immunity. Nat. Rev. Cancer 23, 654–654 (2023).
Su, R. et al. R-2HG exhibits anti-tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell 172, 90–105.e23 (2018).
Li, F., Zhao, D., Wu, J. & Shi, Y. Structure of the YTH domain of human YTHDF2 in complex with an m6A mononucleotide reveals an aromatic cage for m6A recognition. Cell Res. 24, 1490–1492 (2014).
Patil, D. P. et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537, 369–373 (2016).
Liu, J. et al. N6-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science 367, 580–586 (2020).
Widagdo, J., Anggono, V. & Wong, J. J.-L. The multifaceted effects of YTHDC1-mediated nuclear m6A recognition. Trends Genet. 38, 325–332 (2022).
Liu, J. et al. YTHDF2/3 are required for somatic reprogramming through different RNA deadenylation pathways. Cell Rep. 32, 108120 (2020).
Chen, Y. et al. ALKBH5 suppresses malignancy of hepatocellular carcinoma via m6A-guided epigenetic inhibition of LYPD1. Mol. Cancer 19, 123 (2020).
Xue, M. et al. MFAP2, upregulated by m1A methylation, promotes colorectal cancer invasiveness via CLK3. Cancer Med. 12, 8403–8414 (2023).
Yang, J. et al. Mapping of complete set of ribose and base modifications of yeast rRNA by RP-HPLC and mung bean nuclease assay. PLoS ONE 11, e0168873 (2016).
Acknowledgements
The authors thank G. Moshkovitz for drawing the figures. This work was supported by the infrastructure grant of the Israel Innovation Authority, Israel Ministry of Health and the National Headquarter ‘Digital Israel’. The authors also thank the Kahn Family Foundation for continuous support of their research. D.D. is supported by grants from the Israel Science Foundation (2494/18 and 2625/17) and the Human Frontier Science Program (CDA 00048/2018). G.R. is supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 743168) and the Flight Attendant Medical Research Institute (FAMRI). G.R. and D.D. are supported by the German–Israeli Project Cooperation (DIP) of the German Federal Ministry of Education and Research and by a grant from the Varda and Boaz Dotan Research Center in Hemato-Oncology, Tel Aviv University. G.R. holds the Djerassi Chair in Oncology at the Tel Aviv University.
Author information
Authors and Affiliations
Contributions
Introduction (G.R., S.M.-M., M.S.-S., and D.D.); Experimentation (S.M.-M., E.G.-S. and M.S.-S.); Results (E.G.-S., R.A.-F. and S.M.-M.); Applications (S.M.-M. and G.R.); Reproducibility and data deposition (R.A.-F.); Limitations and optimizations (S.M.-M. and G.R.); Outlook (S.M.-M. and G.R.); overview of the Primer (S.M.-M., G.R. and D.D.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Arne Klungland, Guan-Zheng Luo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
European Nucleotide Archive: https://www.ebi.ac.uk/ena/browser/submit
FastQC: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
Gene Expression Omnibus: https://www.ncbi.nlm.nih.gov/geo/
Picard MarkDuplicates: https://broadinstitute.github.io/picard/
Sequence Read Archive: https://www.ncbi.nlm.nih.gov/sra
Trim Galore: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moshitch-Moshkovitz, S., Sevilla-Sharon, M., Ashwal-Fluss, R. et al. mRNA m6A detection. Nat Rev Methods Primers 4, 87 (2024). https://doi.org/10.1038/s43586-024-00365-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-024-00365-9