Abstract
Frailty in aging marks a state of decreased reserves resulting in increased vulnerability to adverse outcomes when exposed to stressors. This Perspective synthesizes the evidence on the aging-related pathophysiology underpinning the clinical presentation of physical frailty as a phenotype of a clinical syndrome that is distinct from the cumulative-deficit-based frailty index. We focus on integrating the converging evidence on the conceptualization of physical frailty as a state, largely independent of chronic diseases, that emerges when the dysregulation of multiple interconnected physiological and biological systems crosses a threshold to critical dysfunction, severely compromising homeostasis. Our exegesis posits that the physiology underlying frailty is a critically dysregulated complex dynamical system. This conceptual framework implies that interventions such as physical activity that have multisystem effects are more promising to remedy frailty than interventions targeted at replenishing single systems. We then consider how this framework can drive future research to further understanding, prevention and treatment of frailty, which will likely preserve health and resilience in aging populations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fried, L. P. et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M157 (2001).
Bandeen-Roche, K. et al. Phenotype of frailty: characterization in the Women’s Health and Aging Studies. J. Gerontol. A Biol. Sci. Med. Sci. 61, 262–266 (2006).
Mitnitski, A. B. et al. Accumulation of deficits as a proxy measure of aging. Sci. World J. 1, 323–336 (2001).
Rockwood, K. & Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 62, 722–727 (2007).
Xue, Q. L. et al. Discrepancy in frailty identification: move beyond predictive validity. J. Gerontol. A Biol. Sci. Med. Sci. 75, 387–393 (2019).
Kelaiditi, E. et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J. Nutr. Health Aging 17, 726–734 (2013).
Xue, Q. L., Buta, B., Ma, L. N., Ge, M. L. & Carlson, M. C. Integrating frailty and cognitive phenotypes: why, how, now what? Curr. Geriatr. Rep. 8, 97–106 (2019).
Bilotta, C. et al. Frailty syndrome diagnosed according to the Study of Osteoporotic Fractures criteria and mortality in older outpatients suffering from Alzheimer’s disease: a one-year prospective cohort study. Aging Ment. Health 16, 273–280 (2012).
Collard, R. M., Boter, H., Schoevers, R. A. & Oude Voshaar, R. C. Prevalence of frailty in community-dwelling older persons: a systematic review. J. Am. Geriatr. Soc. 60, 1487–1492 (2012).
Siriwardhana, D. D., Hardoon, S., Rait, G., Weerasinghe, M. C. & Walters, K. R. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open 8, e018195 (2018).
Llibre Rodriguez, J. J. et al. The prevalence and correlates of frailty in urban and rural populations in Latin America, China, and India: a 10/66 population-based survey. J. Am. Med. Dir. Assoc. 19, 287–295 (2018).
Boyd, C. M., Xue, Q. L., Simpson, C. F., Guralnik, J. M. & Fried, L. P. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am. J. Med. 118, 1225–1231 (2005).
Makary, M. A. et al. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 210, 901–908 (2010).
Bandeen-Roche, K. et al. Principles and issues for physical frailty measurement and its clinical application. J. Gerontol. A Biol. Sci. Med. Sci. 75, 1107–1112 (2020).
Walston, J. et al. Moving frailty toward clinical practice: NIA intramural frailty science symposium summary. J. Am. Geriatr. Soc. 67, 1559–1564 (2019).
Fried, L. P., Ferrucci, L., Darer, J., Williamson, J. D. & Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 59, 255–263 (2004).
Shimokata, H. et al. Age as independent determinant of glucose tolerance. Diabetes 40, 44–51 (1991).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Simon, H. A. The architecture of complexity. Proc. Am. Philos. Soc. 106, 467–482 (1962).
Li, Q. et al. Homeostatic dysregulation proceeds in parallel in multiple physiological systems. Aging Cell 14, 1103–1112 (2015).
Kalyani, R. R., Varadhan, R., Weiss, C. O., Fried, L. P. & Cappola, A. R. Frailty status and altered glucose–insulin dynamics. J. Gerontol. A Biol. Sci. Med. Sci. 67, 1300–1306 (2012).
Blaum, C. S. et al. Is hyperglycemia associated with frailty status in older women? J. Am. Geriatr. Soc. 57, 840–847 (2009).
Perez-Tasigchana, R. F. et al. Metabolic syndrome and insulin resistance are associated with frailty in older adults: a prospective cohort study. Age Ageing 46, 807–812 (2017).
Kalyani, R. R., Varadhan, R., Weiss, C. O., Fried, L. P. & Cappola, A. R. Frailty status and altered dynamics of circulating energy metabolism hormones after oral glucose in older women. J. Nutr. Health Aging 16, 679–686 (2012).
Serra-Prat, M., Palomera, E., Clave, P. & Puig-Domingo, M. Effect of age and frailty on ghrelin and cholecystokinin responses to a meal test. Am. J. Clin. Nutr. 89, 1410–1417 (2009).
Lana, A., Valdés-Bécares, A., Buño, A., Rodríguez-Artalejo, F. & Lopez-Garcia, E. Serum leptin concentration is associated with incident frailty in older adults. Aging Dis. 8, 240–249 (2017).
Ma, L., Sha, G., Zhang, Y. & Li, Y. Elevated serum IL-6 and adiponectin levels are associated with frailty and physical function in Chinese older adults. Clin. Interv. Aging 13, 2013–2020 (2018).
Akki, A. et al. Skeletal muscle ATP kinetics are impaired in frail mice. Age 36, 21–30 (2014).
Ashar, F. N. et al. Association of mitochondrial DNA levels with frailty and all-cause mortality. J. Mol. Med. 93, 177–186 (2015).
Moore, A. Z. et al. Polymorphisms in the mitochondrial DNA control region and frailty in older adults. PLoS ONE 5, e11069 (2010).
Van Epps, P. et al. Frailty has a stronger association with inflammation than age in older veterans. Immun. Ageing 13, 27 (2016).
Bektas, A., Schurman, S. H., Sen, R. & Ferrucci, L. Aging, inflammation and the environment. Exp. Gerontol. 105, 10–18 (2018).
Leng, S. X., Xue, Q.-L., Tian, J., Walston, J. D. & Fried, L. P. Inflammation and frailty in older women. J. Am. Geriatr. Soc. 55, 864–871 (2007).
Walston, J. et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch. Intern. Med. 162, 2333–2341 (2002).
Laudisio, A. et al. The association of olfactory dysfunction, frailty, and mortality is mediated by inflammation: results from the InCHIANTI Study. J. Immunol. Res. 2019, 3128231 (2019).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Bandeen-Roche, K., Walston, J. D., Huang, Y., Semba, R. D. & Ferrucci, L. Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenation Res. 12, 403–410 (2009).
Morrisette-Thomas, V. et al. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech. Ageing Dev. 139, 49–57 (2014).
Soysal, P. et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res. Rev. 31, 1–8 (2016).
Varadhan, R. et al. Frailty and impaired cardiac autonomic control: new insights from principal components aggregation of traditional heart rate variability indices. J. Gerontol. A Biol. Sci. Med. Sci. 64, 682–687 (2009).
Chaves, P. H. M. et al. Physiological complexity underlying heart rate dynamics and frailty status in community-dwelling older women. J. Am. Geriatr. Soc. 56, 1698–1703 (2008).
Lipsitz, L. A. & Goldberger, A. L. Loss of ‘complexity’ and aging: potential applications of fractals and chaos theory to senescence. JAMA 267, 1806–1809 (1992).
Romero-Ortuno, R., Cogan, L., Foran, T., Kenny, R. A. & Fan, C. W. Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people. J. Am. Geriatr. Soc. 59, 655–665 (2011).
Parvaneh, S. et al. Regulation of cardiac autonomic nervous system control across frailty statuses: a systematic review. Gerontology 62, 3–15 (2015).
Johar, H. et al. Blunted diurnal cortisol pattern is associated with frailty: a cross-sectional study of 745 participants aged 65 to 90 years. J. Clin. Endocrinol. Metab. 99, E464–E468 (2014).
Varadhan, R. et al. Higher levels and blunted diurnal variation of cortisol in frail older women. J. Gerontol. A Biol. Sci. Med. Sci. 63, 190–195 (2008).
Voznesensky, M., Walsh, S., Dauser, D., Brindisi, J. & Kenny, A. M. The association between dehydroepiandosterone and frailty in older men and women. Age Ageing 38, 401–406 (2009).
Leng, S. X. et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin. Exp. Res. 16, 153–157 (2004).
Varadhan, R., Seplaki, C. L., Xue, Q. L., Bandeen-Roche, K. & Fried, L. P. Stimulus–response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech. Ageing Dev. 129, 666–670 (2008).
Varadhan, R. et al. Relationship of physical frailty to phosphocreatine recovery in muscle after mild exercise stress in the oldest-old women. J. Frailty Aging 8, 162–168 (2019).
Lewsey, S. C. et al. Exercise intolerance and rapid skeletal muscle energetic decline in human age-associated frailty. JCI Insight 5, e141246 (2020).
Le, N. P., Varadhan, R., Fried, L. P. & Cappola, A. R. Cortisol and dehydroepiandrosterone response to adrenocorticotropic hormone and frailty in older women. J. Gerontol. A Biol. Sci. Med. Sci. https://doi.org/10.1093/gerona/glaa134 (2020).
Yao, X. et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine 29, 5015–5021 (2011).
Wieling, W., Krediet, C. T. P., Van Dijk, N., Linzer, M. & Tschakovsky, M. E. Initial orthostatic hypotension: review of a forgotten condition. Clin. Sci. 112, 157–165 (2007).
Kim, K. & Choe, H. K. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech. Ageing Dev. 177, 74–79 (2019).
Nijhout, H. F., Sadre-Marandi, F., Best, J. & Reed, M. C. Systems biology of phenotypic robustness and plasticity. Integr. Comp. Biol. 57, 171–184 (2017).
McEwen, B. S. Stress, adaptation, and disease: allostasis and allostatic load. Ann. N.Y. Acad. Sci. 840, 33–44 (1998).
Bellavance, M. A. & Rivest, S. The HPA–immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 3, 136 (2014).
Ménard, C., Pfau, M. L., Hodes, G. E. & Russo, S. J. Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology 42, 62–80 (2017).
Braun, T. P. & Marks, D. L. The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 6, 12 (2015).
Pedersen, B. K., Steensberg, A. & Schjerling, P. Exercise and interleukin-6. Curr. Opin. Hematol. 8, 137–141 (2001).
Nance, D. M. & Sanders, V. M. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav. Immun. 21, 736–745 (2007).
Kobayashi, K. S. & Flavell, R. A. Shielding the double-edged sword: negative regulation of the innate immune system. J. Leukocyte Biol. 75, 428–433 (2004).
Epstein, F. H. & Reichlin, S. Neuroendocrine–immune interactions. N. Engl. J. Med. 329, 1246–1253 (1993).
Richards, C. D. Innate immune cytokines, fibroblast phenotypes, and regulation of extracellular matrix in lung. J. Interferon Cytokine Res. 37, 52–61 (2017).
Straub, R. H. Interaction of the endocrine system with inflammation: a function of energy and volume regulation. Arthritis Res. Ther. 16, 203 (2014).
Galoyan, A. Neurochemistry of brain neuroendocrine immune system: signal molecules. Neurochem. Res. 25, 1343–1355 (2000).
Szanton, S. L., Allen, J. K., Seplaki, C. L., Bandeen-Roche, K. & Fried, L. P. Allostatic load and frailty in the Women’s Health and Aging Studies. Biol. Res. Nurs. 10, 248–256 (2009).
Ghachem, A. et al. Evidence from two cohorts for the frailty syndrome as an emergent state of parallel dysregulation in multiple physiological systems. Biogerontology https://doi.org/10.1007/s10522-020-09903-w (2020).
Le Couteur, D. G. & Simpson, S. J. Adaptive senectitude: the prolongevity effects of aging. J. Gerontol. A Biol. Sci. Med. Sci. 66A, 179–182 (2011).
Fulop, T. et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 8, 1960 (2018).
Fried, L. P. et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J. Gerontol. A Biol. Sci. Med. Sci. 64, 1049–1057 (2009).
Kenny, A. M. et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J. Am. Geriatr. Soc. 58, 1134–1143 (2010).
Muller, M., van den Beld, A. W., van der Schouw, Y. T., Grobbee, D. E. & Lamberts, S. W. Effects of dehydroepiandrosterone and atamestane supplementation on frailty in elderly men. J. Clin. Endocrinol. Metab. 91, 3988–3991 (2006).
Nelson, H. D., Walker, M., Zakher, B. & Mitchell, J. Menopausal hormone therapy for the primary prevention of chronic conditions: a systematic review to update the U.S. Preventive Services Task Force recommendations. Ann. Intern. Med. 157, 104–113 (2012).
Scheffer, M. et al. Early-warning signals for critical transitions. Nature 461, 53–59 (2009).
Nakazato, Y. et al. Estimation of homeostatic dysregulation and frailty using biomarker variability: a principal component analysis of hemodialysis patients. Sci. Rep. 10, 10314 (2020).
Gijzel, S. M. et al. Dynamical resilience indicators in time series of self-rated health correspond to frailty levels in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 72, 991–996 (2017).
Yates, F. E. Complexity of a human being: changes with age. Neurobiol. Aging 23, 17–19 (2002).
Xue, Q. L., Bandeen-Roche, K., Varadhan, R., Zhou, J. & Fried, L. P. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J. Gerontol. A Biol. Sci. Med. Sci. 63, 984–990 (2008).
Xue, Q. L., Bandeen-Roche, K., Tian, J., Kasper, J. D. & Fried, L. P. Progression of physical frailty and the risk of all-cause mortality: is there a point of no return? J. Am. Geriatr. Soc. https://doi.org/10.1111/jgs.16976 (2020).
Kirkland, J. L. & Tchkonia, T. Cellular senescence: a translational perspective. EBioMedicine 21, 21–28 (2017).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Andreux, P. A. et al. Mitochondrial function is impaired in the skeletal muscle of pre-frail elderly. Sci. Rep. 8, 8548 (2018).
Ko, F. et al. Impaired mitochondrial degradation by autophagy in the skeletal muscle of the aged female interleukin 10 null mouse. Exp. Gerontol. 73, 23–27 (2016).
Kim, J. A., Wei, Y. & Sowers, J. R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 102, 401–414 (2008).
Mazya, A. L., Garvin, P. & Ekdahl, A. W. Outpatient comprehensive geriatric assessment: effects on frailty and mortality in old people with multimorbidity and high health care utilization. Aging Clin. Exp. Res. 31, 519–525 (2019).
Allen, D. G., Lamb, G. D. & Westerblad, H. Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 88, 287–332 (2008).
Weiss, C. O., Cappola, A. R., Varadhan, R. & Fried, L. P. Resting metabolic rate in old-old women with and without frailty: variability and estimation of energy requirements. J. Am. Geriatr. Soc. 60, 1695–1700 (2012).
Sahin, E. et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365 (2011).
Picard, M. et al. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc. Natl Acad. Sci. USA 112, E6614–E6623 (2015).
Fried, L. P. & Walston, J. in Principles of Geriatric Medicine and Gerontology 4th edn (eds Hazzard, W. R. et al.) 1387–1402 (McGraw Hill, 1998).
Fried, L. P. Interventions for human frailty: physical activity as a model. Cold Spring Harb. Perspect. Med. 6, a025916 (2016).
Bortz, W. Frailty. Mech. Ageing Dev. 129, 680 (2008).
Fried, L. P. et al. A social model for health promotion for an aging population: initial evidence on the Experience Corps model. J. Urban Health 81, 64–78 (2004).
Tan, E. J. et al. The long-term relationship between high-intensity volunteering and physical activity in older African American women. J. Gerontol. B Psychol. Sci. Soc. Sci. 64, 304–311 (2009).
Carlson, M. C. et al. Evidence for neurocognitive plasticity in at-risk older adults: the Experience Corps program. J. Gerontol. A Biol. Sci. Med. Sci. 64, 1275–1282 (2009).
Talegawkar, S. A. et al. A higher adherence to a Mediterranean-style diet is inversely associated with the development of frailty in community-dwelling elderly men and women. J. Nutr. 142, 2161–2166 (2012).
Deer, R. R. & Volpi, E. Protein intake and muscle function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 18, 248–253 (2015).
Fiatarone, M. A. et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 330, 1769–1775 (1994).
Cesari, M. et al. A physical activity intervention to treat the frailty syndrome in older persons—results from the LIFE-P study. J. Gerontol. A Biol. Sci. Med. Sci. 70, 216–222 (2015).
Li, C. M., Chen, C. Y., Li, C. Y., Wang, W. D. & Wu, S. C. The effectiveness of a comprehensive geriatric assessment intervention program for frailty in community-dwelling older people: a randomized, controlled trial. Arch. Gerontol. Geriatr. 50, S39–S42 (2010).
Pazan, F. et al. Current evidence on the impact of medication optimization or pharmacological interventions on frailty or aspects of frailty: a systematic review of randomized controlled trials. Eur. J. Clin. Pharmacol. https://doi.org/10.1007/s00228-020-02951-8 (2020).
Cappola, A. R., Maggio, M. & Ferrucci, L. Is research on hormones and aging finished? No! Just started! J. Gerontol. A Biol. Sci. Med. Sci. 63, 696–697 (2008).
Ma, L. et al. Targeted deletion of interleukin-6 in a mouse model of chronic inflammation demonstrates opposing roles in aging: benefit and harm. J. Gerontol. A Biol. Sci. Med. Sci. https://doi.org/10.1093/gerona/glaa156 (2020).
Fried, L. P. et al. From bedside to bench: research agenda for frailty. Sci. Aging Knowl. Environ. 2005, pe24 (2005).
Chang, S. S., Weiss, C. O., Xue, Q. L. & Fried, L. P. Association between inflammatory-related disease burden and frailty: results from the Women’s Health and Aging Studies (WHAS) I and II. Arch. Gerontol. Geriatr. 54, 9–15 (2012).
Krakauer, D. C. et al. Worlds Hidden in Plain Sight: Thirty Years of Complexity Thinking at the Santa Fe Institute (Santa Fe Institute Press, 2019).
Sterling, P. Allostasis: a model of predictive regulation. Physiol. Behav. 106, 5–15 (2012).
Goldstein, J. Emergence as a construct: history and issues. Emergence 1, 49–72 (1999).
Taffet, G. E. in Geriatric Medicine: An Evidence-Based Approach (eds Cassel, C. K. et al.) 27–28 (Springer Science & Business Media, 2006).
Csete, M. E. & Doyle, J. C. Reverse engineering of biological complexity. Science 295, 1664–1669 (2002).
Strehler, B. L. & Mildvan, A. S. General theory of mortality and aging. Science 132, 14–21 (1960).
Varadhan, R., Walston, J. D. & Bandeen-Roche, K. Can a link be found between physical resilience and frailty in older adults by studying dynamical systems? J. Am. Geriatr. Soc. 66, 1455–1458 (2018).
Kitano, H. Towards a theory of biological robustness. Mol. Syst. Biol. 3, 137 (2007).
Kitano, H. Biological robustness. Nat. Rev. Genet. 5, 826–837 (2004).
Kitano, H. Systems biology: a brief overview. Science 295, 1662–1664 (2002).
Goldstein, J. Emergence in complex systems. In The SAGE Handbook of Complexity and Management (eds Allen, P. et al.) 65–78 (SAGE, 2011).
Bar-Yam, Y. Dynamics of Complex Systems (Routledge, 2019).
Newmann, M., Barabasi, A. L. & Watts, D. J. The Structure and Dynamics of Networks (Princeton Univ. Press, 2006).
Holland, J. H. Complex adaptive systems. Daedalus 121, 17–30 (1992).
Acknowledgements
We dedicate this article to Dr. Richard Suzman, who consistently envisioned and enabled transformative aging research. We are grateful for support by the National Institute on Aging, including for WHAS I (N01 AG012112), WHAS II (M01 R000052), Pathogenesis of Physical Disability in Aging Women (MERIT Award, R37 AG019905) and the frailty-focused Johns Hopkins University Claude D. Pepper Older Americans Independence Center (P30 AG021334). We thank M. A. O’Brien for her outstanding assistance in preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Aging thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fried, L.P., Cohen, A.A., Xue, QL. et al. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging 1, 36–46 (2021). https://doi.org/10.1038/s43587-020-00017-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43587-020-00017-z
This article is cited by
-
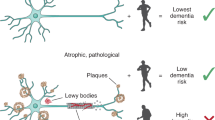
Evaluating the association of physical frailty with cognitive impairment: a clinical perspective in older adults of Bangladesh
BMC Geriatrics (2026)
-
Frailty phenotype reveals heterogeneity in aging and distinct taurine associations
npj Aging (2026)
-
Frailty-related plasma metabolomic signatures predict long-term mortality risk and implicate systemic aging pathways: evidence from a prospective cohort study
npj Aging (2026)
-
Behavioural and psychological factors associated with pre-frailty in community-dwelling adults aged 40 and over: a cross-sectional study
BMC Public Health (2025)
-
Cognitive frailty: a useful concept or a source of confusion? Insights from a survey of European geriatricians
BMC Geriatrics (2025)