Abstract
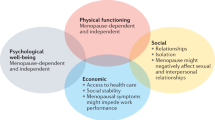
Sex hormone signaling declines during aging, from early midlife through menopause, as a consequence of reduced circulating estrogens and decreased receptiveness to these hormones in target tissues. Estrogens preserve energy homeostasis and promote metabolic health via coordinated and simultaneous effects throughout the brain and body. Age-associated loss of estrogen production during menopause has been implicated in a higher risk for metabolic diseases and increased mortality. However, it remains unclear whether age-associated changes in homeostasis are dependent on reduced estrogen signaling during menopause. Although menopausal hormone therapies containing estrogens can alleviate symptoms, concerns about the risks involved have contributed to a broad decline in the use of these approaches. Non-hormonal therapies have emerged that target tissues or pathways with varying levels of selectivity, reducing risk. We summarize here the broad effects of estrogen loss on homeostasis during menopause, current and emerging therapies and opportunities for understanding homeostatic disruptions associated with menopause.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Dillin, A., Gottschling, D. E. & Nyström, T. The good and the bad of being connected: the integrons of aging. Curr. Opin. Cell Biol. 26, 107–112 (2014).
Pataky, M. W., Young, W. F. & Nair, K. S. Hormonal and metabolic changes of aging and the influence of lifestyle modifications. Mayo Clin. Proc. 96, 788–814 (2021).
Roh, E., Song, D. K. & Kim, M. S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 48, e216 (2016).
Marsh, M. L., Oliveira, M. N. & Vieira-Potter, V. J. Adipocyte metabolism and health after the menopause: the role of exercise. Nutrients 15, 444 (2023).
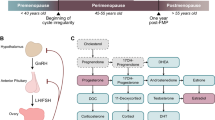
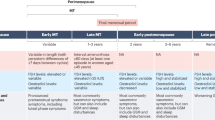
Burger, H. G., Hale, G. E., Dennerstein, L. & Robertson, D. M. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause 15, 603–612 (2008).
Wang, X., Wang, L. & Xiang, W. Mechanisms of ovarian aging in women: a review. J. Ovarian Res. 16, 67 (2023).
Reyes, F. I., Winter, J. S. & Faiman, C. Pituitary–ovarian relationships preceding the menopause. I. A cross-sectional study of serum follice-stimulating hormone, luteinizing hormone, prolactin, estradiol, and progesterone levels. Am. J. Obstet. Gynecol. 129, 557–564 (1977).
Longcope, C., Franz, C., Morello, C., Baker, R. & Johnston, C. C. Steroid and gonadotropin levels in women during the peri-menopausal years. Maturitas 8, 189–196 (1986).
Al-Azzawi, F. & Palacios, S. Hormonal changes during menopause. Maturitas 63, 135–137 (2009).
Ambikairajah, A., Walsh, E., Tabatabaei-Jafari, H. & Cherbuin, N. Fat mass changes during menopause: a metaanalysis. Am. J. Obstet. Gynecol. 221, 393–409 (2019).
Greendale, G. A. et al. Changes in body composition and weight during the menopause transition. JCI Insight 4, e124865 (2019).
Gordon, T., Kannel, W. B., Hjortland, M. C. & McNamara, P. M. Menopause and coronary heart disease. The Framingham Study. Ann. Intern. Med. 89, 157–161 (1978).
Mendelsohn, M. E. Protective effects of estrogen on the cardiovascular system. Am. J. Cardiol. 89, 12–17 (2002).
Recker, R., Lappe, J., Davies, K. & Heaney, R. Characterization of perimenopausal bone loss: a prospective study. J. Bone Miner. Res. 15, 1965–1973 (2000).
Finkelstein, J. S. et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J. Clin. Endocrinol. Metab. 93, 861–868 (2008).
Anagnostis, P. et al. Association between age at menopause and fracture risk: a systematic review and meta-analysis. Endocrine 63, 213–224 (2019).
Dobbs, M. B., Buckwalter, J. & Saltzman, C. Osteoporosis. Iowa Orthop. J. 19, 43–52 (1999).
Sun, L. et al. FSH directly regulates bone mass. Cell 125, 247–260 (2006).
Rocca, W. A., Grossardt, B. R., de Andrade, M., Malkasian, G. D. & Melton, L. J. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 7, 821–828 (2006).
Parker, W. H. et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet. Gynecol. 121, 709–716 (2013).
Gottschau, M. et al. Long-term health consequences after ovarian removal at benign hysterectomy: a nationwide cohort study. Ann. Intern. Med. 176, 596–604 (2023).
Melton, L. J., Crowson, C. S., Malkasian, G. D. & O’Fallon, W. M. Fracture risk following bilateral oophorectomy. J. Clin. Epidemiol. 49, 1111–1115 (1996).
Rocca, W. A. et al. Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health 5, 39–48 (2009).
Rocca, W. A. et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause 15, 1050–1059 (2008).
Jacoby, V. L. et al. Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the Women’s Health Initiative Observational Study. Arch. Intern. Med. 171, 760–768 (2011).
Finch, A. et al. The impact of prophylactic salpingo-oophorectomy on menopausal symptoms and sexual function in women who carry a BRCA mutation. Gynecol. Oncol. 121, 163–168 (2011).
Atsma, F., Bartelink, M. L. E. L., Grobbee, D. E. & van der Schouw, Y. T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause 13, 265–279 (2006).
Mueller, K. & Hsiao, S. Estrus- and ovariectomy-induced body weight changes: evidence for two estrogenic mechanisms. J. Comp. Physiol. Psychol. 94, 1126–1134 (1980).
Rogers, N. H., Perfield, J. W. I. I., Strissel, K. J., Obin, M. S. & Greenberg, A. S. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150, 2161–2168 (2009).
Stubbins, R. E., Holcomb, V. B., Hong, J. & Núñez, N. P. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 51, 861–870 (2012).
Wang, Y. et al. Administration of 17β-estradiol to ovariectomized obese female mice reverses obesity-hypertension through an ACE2-dependent mechanism. Am. J. Physiol. Endocrinol. Metab. 308, E1066–E1075 (2015).
Cintron, D. et al. Plasma orexin A levels in recently menopausal women during and 3 years following use of hormone therapy. Maturitas 99, 59–65 (2017).
Slonaker, J. R. The effect of the excision of different sexual organs on the development, growth and longevity of the albino rat. Am. J. Physiol. 93, 307–317 (1930).
Benedusi, V. et al. Ovariectomy shortens the life span of female mice. Oncotarget 6, 10801–10811 (2015).
Asdell, S. A. & Joshi, S. R. Reproduction and longevity in the hamster and rat. Biol. Reprod. 14, 478–480 (1976).
Phelan, J. P. Reproductive Costs and Longevity in the House Mouse. Ph.D. thesis, Harvard Univ. (1995).
Mühlbock, O. Factors influencing the life-span of inbred mice. Gerontologia 3, 177–183 (2009).
Arriola Apelo, S. I. et al. Ovariectomy uncouples lifespan from metabolic health and reveals a sex-hormone-dependent role of hepatic mTORC2 in aging. eLife 9, e56177 (2020).
Strong, R. et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15, 872–884 (2016).
Harrison, D. E. et al. 17-α-estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell 20, e13328 (2021).
Garratt, M., Bower, B., Garcia, G. G. & Miller, R. A. Sex differences in lifespan extension with acarbose and 17-α estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell 16, 1256–1266 (2017).
Stout, M. B. et al. 17α-estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. J. Gerontol. A Biol. Sci. Med. Sci. 72, 3–15 (2017).
Camon, C. et al. Systemic metabolic benefits of 17α-estradiol are not exclusively mediated by ERα in glutamatergic or GABAergic neurons. Geroscience 46, 6127–6140 (2024).
Garratt, M. et al. 17-α estradiol ameliorates age-associated sarcopenia and improves late-life physical function in male mice but not in females or castrated males. Aging Cell 18, e12920 (2019).
Mann, S. N. et al. Health benefits attributed to 17α-estradiol, a lifespan-extending compound, are mediated through estrogen receptor α. eLife 9, e59616 (2020).
Steyn, F. J. et al. 17α‐estradiol acts through hypothalamic pro‐opiomelanocortin expressing neurons to reduce feeding behavior. Aging Cell 17, e12703 (2018).
Regan, J. C. & Partridge, L. Gender and longevity: why do men die earlier than women? Comparative and experimental evidence. Best Pract. Res. Clin. Endocrinol. Metab. 27, 467–479 (2013).
Austad, S. N. & Fischer, K. E. Sex differences in lifespan. Cell Metab. 23, 1022–1033 (2016).
Miller, R. A. et al. Lifespan effects in male UM-HET3 mice treated with sodium thiosulfate, 16-hydroxyestriol, and late-start canagliflozin. Geroscience 46, 4657–4670 (2024).
Cargill, S. L., Carey, J. R., Müller, H. G. & Anderson, G. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell 2, 185–190 (2003).
Mason, J. B., Cargill, S. L., Anderson, G. B. & Carey, J. R. Transplantation of young ovaries to old mice increased life span in transplant recipients. J. Gerontol. A Biol. Sci. Med. Sci. 64A, 1207–1211 (2009).
Mason, J. B. et al. Transplantation of young ovaries restored cardioprotective influence in postreproductive-aged mice. Aging Cell 10, 448–456 (2011).
Habermehl, T. L. et al. Extension of longevity and reduction of inflammation is ovarian-dependent, but germ cell-independent in post-reproductive female mice. Geroscience 41, 25–38 (2019).
Buffenstein, R., Poppitt, S. D., McDevitt, R. M. & Prentice, A. M. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol. Behav. 58, 1067–1077 (1995).
Fessler, D. M. T. No time to eat: an adaptationist account of periovulatory behavioral changes. Q. Rev. Biol. 78, 3–21 (2003).
Slonaker, J. R. The effect of pubescence, oestruation and menopause on the voluntary activity in the albino rat. Am. J. Physiol. 68, 294–315 (1924).
Brobeck, J. R., Wheatland, M. & Strominger, J. L. Variations in regulation of energy exchange associated with estrus, diestrus and pseudopregnancy in rats. Endocrinology 40, 65–72 (1947).
Kopp, C., Ressel, V., Wigger, E. & Tobler, I. Influence of estrus cycle and ageing on activity patterns in two inbred mouse strains. Behav. Brain Res. 167, 165–174 (2006).
Olofsson, L. E., Pierce, A. A. & Xu, A. W. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc. Natl Acad. Sci. USA 106, 15932–15937 (2009).
Sanchez-Alavez, M., Alboni, S. & Conti, B. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age 33, 89–99 (2011).
Heine, P. A., Taylor, J. A., Iwamoto, G. A., Lubahn, D. B. & Cooke, P. S. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc. Natl Acad. Sci. USA 97, 12729–12734 (2000).
Park, S. et al. Repressor of estrogen receptor activity (REA) is essential for mammary gland morphogenesis and functional activities: studies in conditional knockout mice. Endocrinology 152, 4336–4349 (2011).
Xu, Y. et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 14, 453–465 (2011).
Geary, N., Asarian, L., Korach, K. S., Pfaff, D. W. & Ogawa, S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology 142, 4751–4757 (2001).
Roepke, T. A. et al. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology 149, 6113–6124 (2008).
Martínez de Morentin, P. B. et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 20, 41–53 (2014).
Correa, S. M. et al. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep. 10, 62–74 (2015).
van Veen, J. E. et al. Hypothalamic estrogen receptor α establishes a sexually dimorphic regulatory node of energy expenditure. Nat. Metab. 2, 351–363 (2020).
Krause, W. C. et al. Estrogen drives brain melanocortin to increase physical activity in females. Nature 599, 131–135 (2021).
Spiteri, T., Ogawa, S., Musatov, S., Pfaff, D. W. & Agmo, A. The role of the estrogen receptor α in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behav. Brain Res. 230, 11–20 (2012).
Santollo, J. & Daniels, D. Anorexigenic effects of estradiol in the medial preoptic area occur through membrane-associated estrogen receptors and metabotropic glutamate receptors. Horm. Behav. 107, 20–25 (2019).
Puelles, L. Survey of midbrain, diencephalon, and hypothalamus neuroanatomic terms whose prosomeric definition conflicts with columnar tradition. Front. Neuroanat. 13, 20 (2019).
Xu, P. et al. Estrogen receptor-α in medial amygdala neurons regulates body weight. J. Clin. Invest. 125, 2861–2876 (2015).
Wilson, M. E. et al. Age differentially influences estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) gene expression in specific regions of the rat brain. Mech. Ageing Dev. 123, 593–601 (2002).
Thakur, M. K. & Sharma, P. K. Aging of brain: role of estrogen. Neurochem. Res. 31, 1389–1398 (2006).
Foster, T. C., Sharrow, K. M., Kumar, A. & Masse, J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol. Aging 24, 839–852 (2003).
Hajdarovic, K. H. et al. Single-cell analysis of the aging female mouse hypothalamus. Nat. Aging 2, 662–678 (2022).
Sharma, G. & Prossnitz, E. R. Targeting the G protein-coupled estrogen receptor (GPER) in obesity and diabetes. Endocr. Metab. Sci. 2, 100080 (2021).
Davis, K. E. et al. Sexually dimorphic role of G protein-coupled estrogen receptor (GPER) in modulating energy homeostasis. Horm. Behav. 66, 196–207 (2014).
Della Torre, S. et al. Amino acid-dependent activation of liver estrogen receptor α integrates metabolic and reproductive functions via IGF-1. Cell Metab. 13, 205–214 (2011).
Mondal, S. A. et al. Metabolic benefits of 17α-estradiol in liver are partially mediated by ERβ in male mice. Sci. Rep. 13, 9841 (2023).
Qiu, S. et al. Hepatic estrogen receptor α is critical for regulation of gluconeogenesis and lipid metabolism in males. Sci. Rep. 7, 1661 (2017).
Dahlman, I. et al. Estrogen receptor α gene variants associate with type 2 diabetes and fasting plasma glucose. Pharmacogenet. Genomics 18, 967–975 (2008).
Chow, J. D. Y., Jones, M. E. E., Prelle, K., Simpson, E. R. & Boon, W. C. A selective estrogen receptor α agonist ameliorates hepatic steatosis in the male aromatase knockout mouse. J. Endocrinol. 210, 323–334 (2011).
Della Torre, S. et al. An essential role for liver ERα in coupling hepatic metabolism to the reproductive cycle. Cell Rep. 15, 360–371 (2016).
Francavilla, A. et al. Regenerating rat liver: correlations between estrogen receptor localization and deoxyribonucleic acid synthesis. Gastroenterology 86, 552–557 (1984).
Srisowanna, N. et al. The effect of estrogen on hepatic fat accumulation during early phase of liver regeneration after partial hepatectomy in rats. Acta Histochem. Cytochem. 52, 67–75 (2019).
Kim, S. E., Min, J. S., Lee, S., Lee, D. Y. & Choi, D. Different effects of menopausal hormone therapy on non-alcoholic fatty liver disease based on the route of estrogen administration. Sci. Rep. 13, 15461 (2023).
Mattsson, L. A. et al. Efficacy and tolerability of continuous combined hormone replacement therapy in early postmenopausal women. Menopause Int. 13, 124–131 (2007).
Wang, Y., Wang, Y., Liu, L. & Cui, H. Ovariectomy induces abdominal fat accumulation by improving gonadotropin-releasing hormone secretion in mouse. Biochem. Biophys. Res. Commun. 588, 111–117 (2022).
Nishio, E. et al. Lack of association of ovariectomy-induced obesity with overeating and the reduction of physical activities. Biochem. Biophys. Rep. 20, 100671 (2019).
Camara, C. et al. Effect of ovariectomy on serum adiponectin levels and visceral fat in rats. J. Huazhong Univ. Sci. Technolog. Med. Sci. 34, 825–829 (2014).
Davis, K. E. et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol. Metab. 2, 227–242 (2013).
Richard, A. J., White, U., Elks, C. M. & Stephens, J. M. Adipose tissue: physiology to metabolic dysfunction. In Endotext (eds Feingold, K. R. et al.) (MDText.com, 2000).
Trayhurn, P. & Beattie, J. H. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 60, 329–339 (2001).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Carpentier, A. C. et al. Brown adipose tissue energy metabolism in humans. Front. Endocrinol. 9, 447 (2018).
Heilbronn, L. K. et al. Effect of 6-mo. calorie restriction on biomarkers of longevity, metabolic adaptation and oxidative stress in overweight subjects. JAMA 295, 1539–1548 (2006).
Neff, L. M. et al. Core body temperature is lower in postmenopausal women than premenopausal women: potential implications for energy metabolism and midlife weight gain. Cardiovasc. Endocrinol. 5, 151–154 (2016).
Torres Irizarry, V. C., Jiang, Y., He, Y. & Xu, P. Hypothalamic estrogen signaling and adipose tissue metabolism in energy homeostasis. Front. Endocrinol. 13, 898139 (2022).
Santos, R. S. et al. Activation of estrogen receptor α induces beiging of adipocytes. Mol. Metab. 18, 51–59 (2018).
Koźniewski, K. et al. Epigenetic regulation of estrogen receptor genes’ expressions in adipose tissue in the course of obesity. Int. J. Mol. Sci. 23, 5989 (2022).
Lapid, K., Lim, A., Clegg, D. J., Zeve, D. & Graff, J. M. Oestrogen signalling in white adipose progenitor cells inhibits differentiation into brown adipose and smooth muscle cells. Nat. Commun. 5, 5196 (2014).
Schaffler, M. B. & Kennedy, O. D. Osteocyte signaling in bone. Curr. Osteoporos. Rep. 10, 118–125 (2012).
Matsuoka, K., Park, K., Ito, M., Ikeda, K. & Takeshita, S. Osteoclast-derived complement component 3a stimulates osteoblast differentiation. J. Bone Miner. Res. 29, 1522–1530 (2014).
Bonewald, L. F. & Mundy, G. R. Role of transforming growth factor β in bone remodeling: a review. Connect. Tissue Res. 23, 201–208 (1989).
Cauley, J. A. Estrogen and bone health in men and women. Steroids 99, 11–15 (2015).
Ji, M. X. & Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 1, 9–13 (2015).
Cheng, C. H., Chen, L. R. & Chen, K. H. Osteoporosis due to hormone imbalance: an overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. Int. J. Mol. Sci. 23, 1376 (2022).
Sioka, C. et al. Age at menarche, age at menopause and duration of fertility as risk factors for osteoporosis. Climacteric 13, 63–71 (2010).
Couse, J. F., Lindzey, J., Grandien, K., Gustafsson, J. Å. & Korach, K. S. Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology 138, 4613–4621 (1997).
Wiik, A., Ekman, M., Johansson, O., Jansson, E. & Esbjörnsson, M. Expression of both oestrogen receptor α and β in human skeletal muscle tissue. Histochem. Cell Biol. 131, 181–189 (2009).
Hevener, A. L., Ribas, V., Moore, T. M. & Zhou, Z. The impact of skeletal muscle ERα on mitochondrial function and metabolic health. Endocrinology 161, bqz017 (2020).
Ribas, V. et al. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci. Transl. Med. 8, 334ra54 (2016).
Torres, M. J. et al. 17β-estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab. 27, 167–179 (2018).
Hansen, R. D., Raja, C., Baber, R. J., Lieberman, D. & Allen, B. J. Effects of 20-mg oestradiol implant therapy on bone mineral density, fat distribution and muscle mass in postmenopausal women. Acta Diabetol. 40, s191–s195 (2003).
Ronkainen, P. H. A. et al. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J. Appl. Physiol. 107, 25–33 (2009).
Erkkola, R. et al. Transdermal oestrogen replacement therapy in a Finnish population. Maturitas 13, 275–281 (1991).
Scharf, M. B., McDannold, M. D., Stover, R., Zaretsky, N. & Berkowitz, D. V. Effects of estrogen replacement therapy on rates of cyclic alternating patterns and hot-flush events during sleep in postmenopausal women: a pilot study. Clin. Ther. 19, 304–311 (1997).
Banks, E., Beral, V., Reeves, G., Balkwill, A., Barnes, I. & Million Women Study Collaborators. Fracture incidence in relation to the pattern of use of hormone therapy in postmenopausal women. JAMA 291, 2212–2220 (2004).
Cameron, C. R., Cohen, S., Sewell, K. & Lee, M. The art of hormone replacement therapy (HRT) in menopause management. J. Pharm. Pract. 37, 736–740 (2023).
Notelovitz, M. et al. Initial 17β-estradiol dose for treating vasomotor symptoms. Obstet. Gynecol. 95, 726–731 (2000).
MacLennan, A., Lester, S. & Moore, V. Oral oestrogen replacement therapy versus placebo for hot flushes. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD002978 (2001).
Goldstein, I. Recognizing and treating urogenital atrophy in postmenopausal women. J. Womens Health 19, 425–432 (2010).
de Novaes Soares, C., Almeida, O. P., Joffe, H. & Cohen, L. S. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch. Gen. Psychiatry 58, 529–534 (2001).
Costa, G. B. C., Carneiro, G., Umeda, L., Pardini, D. & Zanella, M. T. Influence of menopausal hormone therapy on body composition and metabolic parameters. Biores. Open Access. 9, 80–85 (2020).
Cappelletti, M. & Wallen, K. Increasing women’s sexual desire: the comparative effectiveness of estrogens and androgens. Horm. Behav. 78, 178–193 (2016).
Cheng, Y. S. et al. Pharmacologic and hormonal treatments for menopausal sleep disturbances: a network meta-analysis of 43 randomized controlled trials and 32,271 menopausal women. Sleep Med. Rev. 57, 101469 (2021).
Pan, Z. et al. Different regimens of menopausal hormone therapy for improving sleep quality: a systematic review and meta-analysis. Menopause 29, 627–635 (2022).
Lufkin, E. G. et al. Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann. Intern. Med. 117, 1–9 (1992).
Lees, B. & Stevenson, J. C. The prevention of osteoporosis using sequential low-dose hormone replacement therapy with estradiol-17β and dydrogesterone. Osteoporos. Int. 12, 251–258 (2001).
Cagnacci, A. & Venier, M. The controversial history of hormone replacement therapy. Medicina 55, 602 (2019).
Buist, D. S. M. et al. Hormone therapy prescribing patterns in the United States. Obstet. Gynecol. 104, 1042–1050 (2004).
Clanget, C. et al. Patterns of hormone replacement therapy in a population-based cohort of postmenopausal German women. Changes after HERS II and WHI. Exp. Clin. Endocrinol. Diabetes 113, 529–533 (2005).
Guay, M. P., Dragomir, A., Pilon, D., Moride, Y. & Perreault, S. Changes in pattern of use, clinical characteristics and persistence rate of hormone replacement therapy among postmenopausal women after the WHI publication. Pharmacoepidemiol. Drug Saf. 16, 17–27 (2007).
Menon, U. et al. Decline in use of hormone therapy among postmenopausal women in the United Kingdom. Menopause 14, 462–467 (2007).
Ettinger, B. et al. Evolution of postmenopausal hormone therapy between 2002 and 2009. Menopause 19, 610–615 (2012).
Mikkola, T. S. et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J. Clin. Endocrinol. Metab. 100, 4588–4594 (2015).
Park, C. Y., Lim, J. Y., Kim, W. H., Kim, S. Y. & Park, H. Y. Evaluation of menopausal hormone therapy use in Korea (2002–2013): a nationwide cohort study. Maturitas 146, 57–62 (2021).
Clarkson, T. B., Meléndez, G. C. & Appt, S. E. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause 20, 342–353 (2013).
El Khoudary, S. R. et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation 142, e506–e532 (2020).
Hodis, H. N. et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N. Engl. J. Med. 374, 1221–1231 (2016).
Ali, N. et al. The role of estrogen therapy as a protective factor for Alzheimer’s disease and dementia in postmenopausal women: a comprehensive review of the literature. Cureus 15, e43053 (2023).
Chlebowski, R. T. et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA 289, 3243–3253 (2003).
Gold, E. B. et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation. Am. J. Public Health 96, 1226–1235 (2006).
Appling, S., Paez, K. & Allen, J. Ethnicity and vasomotor symptoms in postmenopausal women. J. Womens Health 16, 1130–1138 (2007).
DeBono, N. L. et al. Race, menopausal hormone therapy, and invasive breast cancer in the Carolina Breast Cancer Study. J. Womens Health 27, 377–386 (2018).
Cui, J., Shen, Y. & Li, R. Estrogen synthesis and signaling pathways during ageing: from periphery to brain. Trends Mol. Med. 19, 197–209 (2013).
Hou, N. et al. Hormone replacement therapy and breast cancer: heterogeneous risks by race, weight, and breast density. J. Natl Cancer Inst. 105, 1365–1372 (2013).
Chlebowski, R. T. et al. Estrogen alone and health outcomes in Black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause 24, 133–141 (2017).
Graham, S., Archer, D. F., Simon, J. A., Ohleth, K. M. & Bernick, B. Review of menopausal hormone therapy with estradiol and progesterone versus other estrogens and progestins. Gynecol. Endocrinol. 38, 891–910 (2022).
Gompel, A. & Stuenkel, C. A. Neurokinin 3 receptor antagonists for menopausal vasomotor symptoms, an appraisal. Cell Rep. Med. 4, 101076 (2023).
Navarro, V. M. et al. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 29, 11859–11866 (2009).
Clarkson, J. et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc. Natl Acad. Sci. USA 114, E10216–E10223 (2017).
Rance, N. E., Dacks, P. A., Mittelman-Smith, M. A., Romanovsky, A. A. & Krajewski-Hall, S. J. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front. Neuroendocrinol. 34, 211–227 (2013).
David, P. S., Smith, T. L., Nordhues, H. C. & Kling, J. M. A clinical review on paroxetine and emerging therapies for the treatment of vasomotor symptoms. Int. J. Womens Health 14, 353–361 (2022).
Chen, M. N., Lin, C. C. & Liu, C. F. Efficacy of phytoestrogens for menopausal symptoms: a meta-analysis and systematic review. Climacteric 18, 260–269 (2015).
Patisaul, H. B. & Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 31, 400–419 (2010).
Martinkovich, S., Shah, D., Planey, S. L. & Arnott, J. A. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin. Interv. Aging 9, 1437–1452 (2014).
Júnior, J. K., Kulak, C. A. M. & Taylor, H. S. SERMs in the prevention and treatment of postmenopausal osteoporosis: an update. Arq. Bras. Endocrinol. Metabol. 54, 200–205 (2010).
Emons, G., Mustea, A. & Tempfer, C. Tamoxifen and endometrial cancer: a Janus-headed drug. Cancers 12, 2535 (2020).
Mortimer, J. E. et al. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res. Treat. 108, 421–426 (2008).
Balfour, J. A. & Goa, K. L. Raloxifene. Drugs Aging 12, 335–341 (1998).
Mosca, L. et al. Effect of raloxifene on stroke and venous thromboembolism according to subgroups in postmenopausal women at increased risk of coronary heart disease. Stroke 40, 147–155 (2009).
Palacios, S. et al. Raloxifene is not associated with biologically relevant changes in hot flushes in postmenopausal women for whom therapy is appropriate. Am. J. Obstet. Gynecol. 191, 121–131 (2004).
Lello, S., Capozzi, A. & Scambia, G. The tissue-selective estrogen complex (bazedoxifene/conjugated estrogens) for the treatment of menopause. Int. J. Endocrinol. 2017, 5064725 (2017).
Pinkerton, J. V., Pickar, J. H., Racketa, J. & Mirkin, S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric 15, 411–418 (2012).
Shin, J. J., Kim, S. K., Lee, J. R. & Suh, C. S. Ospemifene: a novel option for the treatment of vulvovaginal atrophy. J. Menopausal Med. 23, 79–84 (2017).
Goldstein, S. R. et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet. Gynecol. 95, 95–103 (2000).
Prokai, L. et al. The prodrug DHED selectively delivers 17β-estradiol to the brain for treating estrogen-responsive disorders. Sci. Transl. Med. 7, 297ra113 (2015).
Salinero, A. E. et al. Treatment with brain specific estrogen prodrug ameliorates cognitive effects of surgical menopause in mice. Hormones Behav. 164, 105594 (2024).
Madak-Erdogan, Z. et al. Design of pathway-preferential estrogens that provide beneficial metabolic and vascular effects without stimulating reproductive tissues. Sci. Signal. 9, ra53 (2016).
Zuo, Q. et al. Pathway preferential estrogens prevent hepatosteatosis due to ovariectomy and high-fat diets. Nutrients 13, 3334 (2021).
Gérard, C. & Foidart, J. M. Estetrol: from preclinical to clinical pharmacology and advances in the understanding of the molecular mechanism of action. Drugs R. D. 23, 77–92 (2023).
Holinka, C. F., Brincat, M. & Coelingh Bennink, H. J. T. Preventive effect of oral estetrol in a menopausal hot flush model. Climacteric 11, 15–21 (2008).
Gaspard, U. et al. A multicenter, randomized study to select the minimum effective dose of estetrol (E4) in postmenopausal women (E4Relief): part 1. Vasomotor symptoms and overall safety. Menopause 27, 848–857 (2020).
Takahashi, K. et al. Efficacy and safety of oral estriol for managing postmenopausal symptoms. Maturitas 34, 169–177 (2000).
Griesser, H., Skonietzki, S., Fischer, T., Fielder, K. & Suesskind, M. Low dose estriol pessaries for the treatment of vaginal atrophy: a double-blind placebo-controlled trial investigating the efficacy of pessaries containing 0.2 mg and 0.03 mg estriol. Maturitas 71, 360–368 (2012).
Sánchez-Rovira, P., Hirschberg, A. L., Gil-Gil, M., Bermejo-De Las Heras, B. & Nieto-Magro, C. A phase II prospective, randomized, double-blind, placebo-controlled and multicenter clinical trial to assess the safety of 0.005% estriol vaginal gel in hormone receptor-positive postmenopausal women with early stage breast cancer in treatment with aromatase inhibitor in the adjuvant setting. Oncologist 25, e1846–e1854 (2020).
Abdi, F., Mobedi, H., Mosaffa, N., Dolatian, M. & Ramezani Tehrani, F. Hormone therapy for relieving postmenopausal vasomotor symptoms: a systematic review. Arch. Iran. Med. 19, 141–146 (2016).
Lovre, D., Lindsey, S. H. & Mauvais-Jarvis, F. Effect of menopausal hormone therapy on components of the metabolic syndrome. Ther. Adv. Cardiovasc. Dis. 11, 33–43 (2016).
Raz, L. et al. Differential effects of hormone therapy on serotonin, vascular function and mood in the KEEPS. Climacteric 19, 49–59 (2016).
Fait, T. Menopause hormone therapy: latest developments and clinical practice. Drugs Context 8, 212551 (2019).
Gosset, A., Pouillès, J. M. & Trémollieres, F. Menopausal hormone therapy for the management of osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 35, 101551 (2021).
Meziou, N., Scholfield, C., Taylor, C. A. & Armstrong, H. L. Hormone therapy for sexual function in perimenopausal and postmenopausal women: a systematic review and meta-analysis update. Menopause 30, 659–671 (2023).
Wright, A. C. et al. The effectiveness and value of fezolinetant for moderate-to-severe vasomotor symptoms associated with menopause: a summary from the Institute for Clinical and Economic Review’s Midwest Public Advisory Council. J. Manag. Care Spec. Pharm. 29, 692–698 (2023).
Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).
Clark, J. H. A critique of Women’s Health Initiative studies (2002-2006). Nucl. Recept. Signal. 4, e023 (2006).
Manson, J. E. et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the women’s health initiative randomized trials. JAMA 310, 1353–1368 (2013).
Grady, D. et al. Heart and Estrogen/progestin Replacement Study (HERS): design, methods, and baseline characteristics. Control. Clin. Trials. 19, 314–335 (1998).
Herrington, D. M. The HERS trial results: paradigms lost? Heart and Estrogen/progestin Replacement Study. Ann. Intern. Med. 131, 463–466 (1999).
El Khoudary, S. R. et al. The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN). Menopause 26, 1213–1227 (2019).
Kravitz, H. M. et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause 10, 19–28 (2003).
Matthews, K. A. et al. Associations between depressive symptoms and inflammatory/hemostatic markers in women during the menopausal transition. Psychosom. Med. 69, 124–130 (2007).
Hodis, H. N. et al. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause 22, 391–401 (2015).
Miller, V. M. et al. The Kronos Early Estrogen Prevention Study (KEEPS): what have we learned? Menopause 26, 1071–1084 (2019).
Mosekilde, L. et al. The Danish Osteoporosis Prevention Study (DOPS): project design and inclusion of 2000 normal perimenopausal women. Maturitas 31, 207–219 (1999).
Schierbeck, L. L. et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 345, e6409 (2012).
Shufelt, C. L. & Manson, J. E. Menopausal hormone therapy and cardiovascular disease: the role of formulation, dose, and route of delivery. J. Clin. Endocrinol. Metab. 106, 1245–1254 (2021).
Rejnmark, L. et al. Response rates to oestrogen treatment in perimenopausal women: 5-year data from the Danish Osteoporosis Prevention Study (DOPS). Maturitas 48, 307–320 (2004).
Karim, R. et al. Determinants of the effect of estrogen on the progression of subclinical atherosclerosis: Estrogen in the Prevention of Atherosclerosis Trial. Menopause 12, 366–373 (2005).
Straczek, C. et al. Prothrombotic mutations, hormone therapy, and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration. Circulation 112, 3495–3500 (2005).
Hodis, H. N. et al. Estrogen in the prevention of atherosclerosis. a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 135, 939–953 (2001).
Mosca, L. et al. Hormone replacement therapy and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 104, 499–503 (2001).
Kelemen, M. et al. Hormone therapy and antioxidant vitamins do not improve endothelial vasodilator function in postmenopausal women with established coronary artery disease: a substudy of the Women’s Angiographic Vitamin and Estrogen (WAVE) trial. Atherosclerosis 179, 193–200 (2005).
Waters, D. D. et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA 288, 2432–2440 (2002).
Veerus, P. et al. Results from the Estonian postmenopausal hormone therapy trial [ISRCTN35338757]. Maturitas 55, 162–173 (2006).
Hovi, S. L., Veerus, P., Rahu, M. & Hemminki, E. Experiences of a long-term randomized controlled prevention trial in a maiden environment: Estonian Postmenopausal Hormone Therapy trial. BMC Med. Res. Methodol. 8, 51 (2008).
Yang, D., Li, J., Yuan, Z. & Liu, X. Effect of hormone replacement therapy on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PLoS ONE 8, e62329 (2013).
Stepniak, J. & Karbownik-Lewinska, M. 17β-estradiol prevents experimentally-induced oxidative damage to membrane lipids and nuclear DNA in porcine ovary. Syst. Biol. Reprod. Med. 62, 17–21 (2016).
Malespin, M. & Nassri, A. Endocrine diseases and the liver: an update. Clin. Liver Dis. 23, 233–246 (2019).
Hess, R. A. Estrogen in the adult male reproductive tract: a review. Reprod. Biol. Endocrinol. 1, 52 (2003).
Lee, H. R., Kim, T. H. & Choi, K. C. Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Lab Anim. Res. 28, 71–76 (2012).
Hewitt, S. C., Winuthayanon, W. & Korach, K. S. What’s new in estrogen receptor action in the female reproductive tract. J. Mol. Endocrinol. 56, R55–R71 (2016).
Tang, Z. R., Zhang, R., Lian, Z. X., Deng, S. L. & Yu, K. Estrogen-receptor expression and function in female reproductive disease. Cells 8, 1123 (2019).
Osterlund, M. K., Keller, E. & Hurd, Y. L. The human forebrain has discrete estrogen receptor α messenger RNA expression: high levels in the amygdaloid complex. Neuroscience 95, 333–342 (2000).
Wilson, M. E., Westberry, J. M. & Prewitt, A. K. Dynamic regulation of estrogen receptor-α gene expression in the brain: a role for promoter methylation? Front. Neuroendocrinol. 29, 375–385 (2008).
Liu, X. & Shi, H. Regulation of estrogen receptor α expression in the hypothalamus by sex steroids: implication in the regulation of energy homeostasis. Int. J. Endocrinol. 2015, 949085 (2015).
Shughrue, P. J., Komm, B. & Merchenthaler, I. The distribution of estrogen receptor-β mRNA in the rat hypothalamus. Steroids 61, 678–681 (1996).
Björnström, L. & Sjöberg, M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 19, 833–842 (2005).
Fuentes, N. & Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 116, 135–170 (2019).
Marino, M., Galluzzo, P. & Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genomics 7, 497–508 (2006).
Méndez-Reséndiz, K. A. et al. Steroids and TRP channels: a close relationship. Int. J. Mol. Sci. 21, 3819 (2020).
Acknowledgements
We are grateful for scholarships to C.C. from the Elman Poole Trust, the Hope Foundation for Research on Ageing, the Collaboration of Ageing Research Excellence at the University of Otago and the Australasian Menopause Society. S.M.C. was supported by NIH grants AG066821 and DK136073.
Author information
Authors and Affiliations
Contributions
C.C. led the conceptualization and drafting of the manuscript. C.C., M.G. and S.M.C. drafted and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Miguel López and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Camon, C., Garratt, M. & Correa, S.M. Exploring the effects of estrogen deficiency and aging on organismal homeostasis during menopause. Nat Aging 4, 1731–1744 (2024). https://doi.org/10.1038/s43587-024-00767-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43587-024-00767-0