Abstract
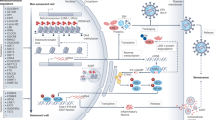
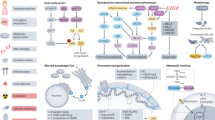
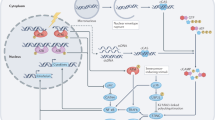
Cancer is an age-related disease, but the interplay between cancer and aging is complex and their shared molecular drivers are deeply intertwined. This Review provides an overview of how different biological pathways affect cancer and aging, leveraging evidence mainly derived from animal studies. We discuss how genome maintenance and accumulation of DNA mutations affect tumorigenesis and tissue homeostasis during aging. We describe how age-related telomere dysfunction and cellular senescence intricately modulate tumor development through mechanisms involving genomic instability and inflammation. We examine how an aged immune system and chronic inflammation shape tumor immunosurveillance, fueling DNA damage and cellular senescence. Finally, as animal models are important to untangling the relative contributions of these aging-modulated pathways to cancer progression and to test interventions, we discuss some of the limitations of physiological and accelerated aging models, aiming to improve experimental designs and enhance translation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
DeSantis, C. E. et al. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J. Clin. 69, 452–467 (2019).
Mattiuzzi, C. & Lippi, G. Current cancer epidemiology. J. Epidemiol. Glob. Health 9, 217–222 (2019).
The importance of aging in cancer research. Nat. Aging 2, 365–366 (2022).
Radkiewicz, C., Kronmark, J. J., Adami, H. O. & Edgren, G. Declining cancer incidence in the elderly: decreasing diagnostic intensity or biology? Cancer Epidemiol. Biomarkers Prev. 31, 280–286 (2022).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Zhao, Y., Simon, M., Seluanov, A. & Gorbunova, V. DNA damage and repair in age-related inflammation. Nat. Rev. Immunol. 23, 75–89 (2023).
Niedernhofer, L. J. et al. Nuclear genomic instability and aging. Annu. Rev. Biochem. 87, 295–322 (2018).
Folgueras, A. R., Freitas-Rodríguez, S., Velasco, G. & López-Otín, C. Mouse models to disentangle the hallmarks of human aging. Circ. Res. 123, 905–924 (2018).
Laconi, E., Marongiu, F. & DeGregori, J. Cancer as a disease of old age: changing mutational and microenvironmental landscapes. Br. J. Cancer 122, 943–952 (2020).
Guarente, L., Sinclair, D. A. & Kroemer, G. Human trials exploring anti-aging medicines. Cell Metab. 36, 354–376 (2024).
Foley, N. M. et al. Growing old, yet staying young: the role of telomeres in bats’ exceptional longevity. Sci. Adv. 4, eaao0926 (2018).
Cagan, A. et al. Somatic mutation rates scale with lifespan across mammals. Nature 604, 517–524 (2022).
Tian, X. et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499, 346–349 (2013).
Seluanov, A., Gladyshev, V. N., Vijg, J. & Gorbunova, V. Mechanisms of cancer resistance in long-lived mammals. Nat. Rev. Cancer 18, 433–441 (2018).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
López-Otín, C., Pietrocola, F., Roiz-Valle, D., Galluzzi, L. & Kroemer, G. Meta-hallmarks of aging and cancer. Cell Metab. 35, 12–35 (2023).
Boveri, T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. J. Cell Sci. 121, 1–84 (2008).
Martincorena, I. & Campbell, P. J. Somatic mutation in cancer and normal cells. Science 349, 1483–1489 (2015).
Campbell, P. J. et al. Pan-cancer analysis of whole genomes. Nature 578, 82–93 (2020).
Maciejowski, J., Li, Y., Bosco, N., Campbell, P. J. & De Lange, T. Chromothripsis and kataegis induced by telomere crisis. Cell 163, 1641–1654 (2015).
Lee-Six, H. et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature 574, 532–537 (2019).
Loeb, L. A. Human cancers express mutator phenotypes: origin, consequences and targeting. Nat. Rev. Cancer 11, 450–457 (2011).
Evans, E. J. & DeGregori, J. Cells with cancer‐associated mutations overtake our tissues as we age. Aging Cancer 2, 82–97 (2021).
Martincorena, I. Somatic mutation and clonal expansions in human tissues. Genome Med. 11, 11–13 (2019).
Li, R., Sonik, A., Stindl, R., Rasnick, D. & Duesberg, P. Aneuploidy vs. gene mutation hypothesis of cancer: recent study claims mutation but is found to support aneuploidy. Proc. Natl Acad. Sci. USA 97, 3236–3241 (2000).
Zhang, L. & Vijg, J. Somatic mutagenesis in mammals and its implications for human disease and aging. Annu. Rev. Genet. 52, 397–419 (2018).
Vijg, J. et al. Mitigating age-related somatic mutation burden. Trends Mol. Med. 29, 530–540 (2023).
Li, C. H., Haider, S. & Boutros, P. C. Age influences on the molecular presentation of tumours. Nat. Commun. 13, 208 (2022).
Smith, A. L. M., Whitehall, J. C. & Greaves, L. C. Mitochondrial DNA mutations in ageing and cancer. Mol. Oncol. 16, 3276–3294 (2022).
Gyenis, A. et al. Genome-wide RNA polymerase stalling shapes the transcriptome during aging. Nat. Genet. 55, 268–279 (2023).
Vijg, J. & Dong, X. Pathogenic mechanisms of somatic mutation and genome mosaicism in aging. Cell 182, 12–23 (2020).
Jonason, A. S. et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc. Natl Acad. Sci. USA 93, 14025–14029 (1996).
Jaiswal, S. & Ebert, B. L. Clonal hematopoiesis in human aging and disease. Science 366, eaan4673 (2019).
Nathan, D. I., Dougherty, M., Bhatta, M., Mascarenhas, J. & Marcellino, B. K. Clonal hematopoiesis and inflammation: a review of mechanisms and clinical implications. Crit. Rev. Oncol. Hematol. 192, 104187 (2023).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Yokoyama, A. et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature 565, 312–317 (2019).
Martincorena, I. et al. Somatic mutant clones colonize the human esophagus with age. Science 362, 911–917 (2018).
Abascal, F. et al. Somatic mutation landscapes at single-molecule resolution. Nature 593, 405–410 (2021).
Colom, B. et al. Mutant clones in normal epithelium outcompete and eliminate emerging tumours. Nature 598, 510–514 (2021).
Abby, E. et al. Notch1 mutations drive clonal expansion in normal esophageal epithelium but impair tumor growth. Nat. Genet. 55, 232–245 (2023).
Panier, S., Wang, S. & Schumacher, B. Genome instability and DNA repair in somatic and reproductive aging. Annu. Rev. Pathol. 24, 261–290 (2023).
Zhang, L. et al. Maintenance of genome sequence integrity in long- and short-lived rodent species. Sci. Adv. 7, eabj3284 (2021).
Tian, X. et al. SIRT6 is responsible for more efficient DNA double-strand break repair in long-lived species. Cell 177, 622–638 (2019).
Tian, X., Seluanov, A. & Gorbunova, V. Molecular mechanisms determining lifespan in short- and long-lived species. Trends Endocrinol. Metab. 28, 722–734 (2017).
Gorbunova, V., Seluanov, A., Mao, Z. & Hine, C. Changes in DNA repair during aging. Nucleic Acids Res. 35, 7466–7474 (2007).
Tomasetti, C., Li, L. & Vogelstein, B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 355, 1330–1334 (2017).
Tomasetti, C. & Vogelstein, B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347, 78–81 (2015).
Rieckher, M., Garinis, G. A. & Schumacher, B. Molecular pathology of rare progeroid diseases. Trends Mol. Med. 27, 907–922 (2021).
Marteijn, J. A., Lans, H., Vermeulen, W. & Hoeijmakers, J. H. J. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 15, 465–481 (2014).
Montégut, L., López-Otín, C. & Kroemer, G. Aging and cancer. Mol. Cancer 23, 106 (2024).
Pérez, R. F., Tejedor, J. R., Fernández, A. F. & Fraga, M. F. Aging and cancer epigenetics: where do the paths fork? Aging Cell 21, e13709 (2022).
Liu, Z. et al. Immunosenescence: molecular mechanisms and diseases. Signal Transduct. Target. Ther. 8, 200 (2023).
Bottazzi, B., Riboli, E. & Mantovani, A. Aging, inflammation and cancer. Semin. Immunol. 40, 74–82 (2018).
Horvath, S. & Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 19, 371–384 (2018).
Wang, S., El Jurdi, N., Thyagarajan, B., Prizment, A. & Blaes, A. H. Accelerated aging in cancer survivors: cellular senescence, frailty, and possible opportunities for interventions. Int. J. Mol. Sci. 25, 3319 (2024).
Perez, K. et al. DNA repair-deficient premature aging models display accelerated epigenetic age. Aging Cell 23, e14058 (2023).
Yang, J. H. et al. Loss of epigenetic information as a cause of mammalian aging. Cell 186, 305–326 (2023).
Lu, A. T. et al. Universal DNA methylation age across mammalian tissues. Nat. Aging 3, 1144–1166 (2023).
Borges, G., Criqui, M. & Harrington, L. Tieing together loose ends: telomere instability in cancer and aging. Mol. Oncol. 16, 3380–3396 (2022).
Csiszar, A. et al. Chemically induced carcinogenesis in rodent models of aging: assessing organismal resilience to genotoxic stressors in geroscience research. Geroscience 41, 209–227 (2019).
De Lange, T. Shelterin-mediated telomere protection. Annu. Rev. Genet. 52, 223–247 (2018).
Rossiello, F., Jurk, D., Passos, J. F. & d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 24, 135–147 (2022).
Chakravarti, D., LaBella, K. A. & DePinho, R. A. Telomeres: history, health, and hallmarks of aging. Cell 184, 306–322 (2021).
D’Adda Di Fagagna, F. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003).
Fumagalli, M. et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 14, 355–365 (2012).
Hewitt, G. et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 3, 708 (2012).
Barnes, R. P. et al. Telomeric 8-oxo-guanine drives rapid premature senescence in the absence of telomere shortening. Nat. Struct. Mol. Biol. 29, 639–652 (2022).
Jurk, D. et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2, 4172 (2014).
Lansdorp, P. M. Telomeres, aging, and cancer: the big picture. Blood 139, 813–821 (2022).
Demanelis, K. et al. Determinants of telomere length across human tissues. Science 369, eaaz6876 (2020).
Suram, A. & Herbig, U. The replicometer is broken: telomeres activate cellular senescence in response to genotoxic stresses. Aging Cell 13, 780–786 (2014).
Nassour, J., Przetocka, S. & Karlseder, J. Telomeres as hotspots for innate immunity and inflammation. DNA Repair 133, 103591 (2024).
Rossiello, F. et al. DNA damage response inhibition at dysfunctional telomeres by modulation of telomeric DNA damage response RNAs. Nat. Commun. 8, 13980 (2017).
Aguado, J. et al. Inhibition of DNA damage response at telomeres improves the detrimental phenotypes of Hutchinson–Gilford progeria syndrome. Nat. Commun. 10, 4990 (2019).
Rosso, I. et al. Alternative lengthening of telomeres (ALT) cells viability is dependent on C-rich telomeric RNAs. Nat. Commun. 14, 7086 (2023).
Nassour, J., Schmidt, T. T. & Karlseder, J. Telomeres and cancer: resolving the paradox. Annu. Rev. Cancer Biol. 5, 59–77 (2020).
Barthel, F. P. et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat. Genet. 49, 349–357 (2017).
De Vitis, M., Berardinelli, F. & Sgura, A. Telomere length maintenance in cancer: at the crossroad between telomerase and alternative lengthening of telomeres (ALT). Int. J. Mol. Sci. 19, 606 (2018).
Claude, E. & Decottignies, A. Telomere maintenance mechanisms in cancer: telomerase, ALT or lack thereof. Curr. Opin. Genet. Dev. 60, 1–8 (2020).
Viceconte, N. et al. Highly aggressive metastatic melanoma cells unable to maintain telomere length. Cell Rep. 19, 2529–2543 (2017).
Dagg, R. A. et al. Extensive proliferation of human cancer cells with ever-shorter telomeres. Cell Rep. 19, 2544–2556 (2017).
Seger, Y. R. et al. Transformation of normal human cells in the absence of telomerase activation. Cancer Cell 2, 401–413 (2002).
Taboski, M. A. S. et al. Long telomeres bypass the requirement for telomere maintenance in human tumorigenesis. Cell Rep. 1, 91–98 (2012).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Byrjalsen, A., Brainin, A. E., Lund, T. K., Andersen, M. K. & Jelsig, A. M. Size matters in telomere biology disorders — expanding phenotypic spectrum in patients with long or short telomeres. Hered. Cancer Clin. Pract. 21, 7 (2023).
Gomes, N. M. V. et al. Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 10, 761–768 (2011).
Henriques, C. M., Carneiro, M. C., Tenente, I. M. & Ferreira, J. A. Telomerase is required for zebrafish lifespan. PLoS Genet. 9, 1003214 (2013).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997).
Liu, N. et al. HTERT promotes tumor angiogenesis by activating VEGF via interactions with the Sp1 transcription factor. Nucleic Acids Res. 44, 8693–8703 (2016).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Jaskelioff, M. et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469, 102–107 (2011).
Lex, K. et al. Telomere shortening produces an inflammatory environment that increases tumor incidence in zebrafish. Proc. Natl Acad. Sci. USA 117, 15066–15074 (2020).
Varela, E., Muñoz-Lorente, M. A., Tejera, A. M., Ortega, S. & Blasco, M. A. Generation of mice with longer and better preserved telomeres in the absence of genetic manipulations. Nat. Commun. 7, 11739 (2016).
Blasco, M. A. Mice with bad ends: mouse models for the study of telomeres and telomerase in cancer and aging. EMBO J. 24, 1095–1103 (2005).
González-Suárez, E., Samper, E., Flores, J. M. & Blasco, M. A. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat. Genet. 26, 114–117 (2000).
Franco, S., Segura, I., Riese, H. H. & Blasco, M. A. Decreased B16F10 melanoma growth and impaired vascularization in telomerase-deficient mice with critically short telomeres. Cancer Res. 62, 552–559 (2002).
Ding, Z. et al. Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell 148, 896–907 (2012).
Platzbecker, U. et al. Imetelstat in patients with lower-risk myelodysplastic syndromes who have relapsed or are refractory to erythropoiesis-stimulating agents (IMerge): a multinational, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 403, 249–260 (2024).
Muñoz-Lorente, M. A. et al. AAV9-mediated telomerase activation does not accelerate tumorigenesis in the context of oncogenic K-Ras-induced lung cancer. PLoS Genet. 14, e1007562 (2018).
Di Micco, R., Krizhanovsky, V., Baker, D. & d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95 (2021).
Dasgupta, N., Arnold, R., Equey, A., Gandhi, A. & Adams, P. D. The role of the dynamic epigenetic landscape in senescence: orchestrating SASP expression. NPJ Aging 10, 48 (2024).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Di Micco, R. et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444, 638–642 (2006).
Liu, J. Y. et al. Cells exhibiting strong p16INK4a promoter activation in vivo display features of senescence. Proc. Natl Acad. Sci. USA 116, 2603–2611 (2019).
Ogata, Y., Yamada, T., Hasegawa, S., Sugiura, K. & Akamatsu, H. Changes of senescent cell accumulation and removal in skin tissue with ageing. Exp. Dermatol. 32, 1159–1161 (2023).
Haston, S. et al. Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell 41, 1242–1260 (2023).
Schafer, M. J. et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight 5, e133668 (2020).
Acosta, J. C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990 (2013).
Victorelli, S. et al. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J. 38, e101982 (2019).
da Silva, P. F. L. et al. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 18, e12848 (2019).
Fane, M. & Weeraratna, A. T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 20, 89–106 (2020).
Chaib, S., Tchkonia, T. & Kirkland, J. L. Cellular senescence and senolytics: the path to the clinic. Nat. Med. 28, 1556–1568 (2022).
Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021).
Wang, T. W. et al. Blocking PD-L1–PD-1 improves senescence surveillance and ageing phenotypes. Nature 611, 358–364 (2022).
Majewska, J. et al. p16-dependent increase of PD-L1 stability regulates immunosurveillance of senescent cells. Nat. Cell Biol. 26, 1336–1345 (2024).
Chaib, S. et al. The efficacy of chemotherapy is limited by intratumoral senescent cells expressing PD-L2. Nat. Cancer 5, 448–462 (2024).
Karin, O., Agrawal, A., Porat, Z., Krizhanovsky, V. & Alon, U. Senescent cell turnover slows with age providing an explanation for the Gompertz law. Nat. Commun. 10, 5495 (2019).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Hernandez-Gonzalez, F. et al. Human senescent fibroblasts trigger progressive lung fibrosis in mice. Aging 15, 6641–6657 (2023).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Baker, D. J. et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Amor, C. et al. Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nat. Aging 4, 336–349 (2024).
Schmitt, C. A., Wang, B. & Demaria, M. Senescence and cancer — role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 19, 619–636 (2022).
Kolodkin-Gal, D. et al. Senolytic elimination of Cox2-expressing senescent cells inhibits the growth of premalignant pancreatic lesions. Gut 71, 345–355 (2022).
Braig, M. et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436, 660–665 (2005).
Collado, M. et al. Tumour biology: senescence in premalignant tumours. Nature 436, 642 (2005).
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005).
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005).
Di Micco, R. et al. Interplay between oncogene-induced DNA damage response and heterochromatin in senescence and cancer. Nat. Cell Biol. 13, 292–302 (2011).
Prieto, L. I. et al. Senescent alveolar macrophages promote early-stage lung tumorigenesis. Cancer Cell 41, 1261–1275 (2023).
Fletcher-Sananikone, E. et al. Elimination of radiation-induced senescence in the brain tumor microenvironment attenuates glioblastoma recurrence. Cancer Res. 81, 5935–5947 (2021).
Liu, D. & Hornsby, P. J. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 67, 3117–3126 (2007).
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001).
Ruhland, M. K. et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 7, 11762 (2016).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017).
Bhakta, N. et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 390, 2569–2582 (2017).
González-Gualda, E. et al. Galacto-conjugation of navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell 19, e13142 (2020).
Fleury, H. et al. Exploiting interconnected synthetic lethal interactions between PARP inhibition and cancer cell reversible senescence. Nat. Commun. 10, 2556 (2019).
Saleh, T. et al. Clearance of therapy-induced senescent tumor cells by the senolytic ABT-263 via interference with BCL-XL–BAX interaction. Mol. Oncol. 14, 2504–2519 (2020).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Nikolich-Žugich, J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 19, 10–19 (2018).
Ikeda, H. & Togashi, Y. Aging, cancer, and antitumor immunity. Int. J. Clin. Oncol. 27, 316–322 (2022).
Fulop, T. et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 8, 1960 (2018).
Arai, Y. et al. Inflammation, but not telomere length, predicts successful ageing at extreme old age: a longitudinal study of semi-supercentenarians. EBioMedicine 2, 1549–1558 (2015).
Gonzalez, H., Hagerling, C. & Werb, Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 32, 1267–1284 (2018).
Matsushita, H. et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482, 400–404 (2012).
Chiodoni, C. et al. Transcriptional profiles and stromal changes reveal bone marrow adaptation to early breast cancer in association with deregulated circulating microRNAs. Cancer Res. 80, 484–498 (2020).
Hill, W. et al. Lung adenocarcinoma promotion by air pollutants. Nature 616, 159–167 (2023).
Johnson, C. C., Hayes, R. B., Schoen, R. E., Gunter, M. J. & Huang, W.-Y. Non-steroidal anti-inflammatory drug use and colorectal polyps in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Gastroenterol. 105, 2646–2655 (2010).
Han, Z., Brown, J. R. & Niederkorn, J. Y. Growth and metastasis of intraocular tumors in aged mice. Invest. Ophthalmol. Vis. Sci. 57, 2366–2376 (2016).
Golomb, L. et al. Age-associated inflammation connects RAS-induced senescence to stem cell dysfunction and epidermal malignancy. Cell Death Differ. 22, 1764–1774 (2015).
Park, M. D. et al. Hematopoietic aging promotes cancer by fueling IL-1α-driven emergency myelopoiesis. Science 386, eadn0327 (2024).
Anczuków, O. et al. Challenges and opportunities for modeling aging and cancer. Cancer Cell 41, 641–645 (2023).
Schratz, K. E. et al. T cell immune deficiency rather than chromosome instability predisposes patients with short telomere syndromes to squamous cancers. Cancer Cell 41, 807–817 (2023).
Blasco, M. A. Immunosenescence phenotypes in the telomerase knockout mouse. Springer Semin. Immunopathol. 24, 75–85 (2002).
Jackson, S. J. et al. Does age matter? The impact of rodent age on study outcomes. Lab. Anim. 51, 160–169 (2017).
Jenkins, E. C. et al. Age alters the oncogenic trajectory toward luminal mammary tumors that activate unfolded proteins responses. Aging Cell 21, e13665 (2022).
Chhabra, Y. et al. Sex-dependent effects in the aged melanoma tumor microenvironment influence invasion and resistance to targeted therapy. Cell 187, 6016–6034 (2024).
Moore, A. L., Shore, H., Ershler, W. B. & Gamelli, R. L. Transfer of age-associated restrained tumor growth in mice by old-to-young bone marrow transplantation. Cancer Res. 44, 5677–5680 (1984).
Scannell, J. W. et al. Predictive validity in drug discovery: what it is, why it matters and how to improve it. Nat. Rev. Drug Discov. 21, 915–931 (2022).
Olson, B., Li, Y., Lin, Y., Liu, E. T. & Patnaik, A. Mouse models for cancer immunotherapy research. Cancer Discov. 8, 1358–1365 (2018).
Bouleftour, W. & Magne, N. Aging preclinical models in oncology field: from cells to aging. Aging Clin. Exp. Res. 34, 751–755 (2022).
Reed, M. J. et al. The effects of aging on tumor growth and angiogenesis are tumor-cell dependent. Int. J. Cancer 120, 753–760 (2007).
Ershler, W. B., Stewart, J. A., Hacker, M. P., Moore, A. L. & Tindle, B. H. B16 murine melanoma and aging: slower growth and longer survival in old mice. J. Natl Cancer Inst. 72, 161–164 (1984).
Pili, R. et al. Altered angiogenesis underlying age-dependent changes in tumor growth. J. Natl Cancer Inst. 86, 1303–1314 (1994).
Kreisle, R. A., Stebler, B. A. & Ershler, W. B. Effect of host age on tumor-associated angiogenesis in mice. J. Natl Cancer Inst. 82, 44–47 (1990).
Skou, S. T. et al. Multimorbidity. Nat. Rev. Dis. Primers 8, 48 (2022).
Sanchez, S. E. et al. Digital telomere measurement by long-read sequencing distinguishes healthy aging from disease. Nat. Commun. 15, 5148 (2024).
Whittemore, K., Vera, E., Martínez-Nevado, E., Sanpera, C. & Blasco, M. A. Telomere shortening rate predicts species life span. Proc. Natl Acad. Sci. USA 116, 15122–15127 (2019).
Vera, E., Bernardes de Jesus, B., Foronda, M., Flores, J. M. & Blasco, M. A. The rate of increase of short telomeres predicts longevity in mammals. Cell Rep. 2, 732–737 (2012).
Lorenzini, A. et al. Significant correlation of species longevity with DNA double strand break recognition but not with telomere length. Mech. Ageing Dev. 130, 784–792 (2009).
Fick, L. J. et al. Telomere length correlates with life span of dog breeds. Cell Rep. 2, 1530–1536 (2012).
Domínguez-de-Barros, A. et al. An approach to the effects of longevity, sexual maturity, and reproduction on telomere length and oxidative stress in different Psittacidae species. Front. Genet. 14, 1156730 (2023).
Buddhachat, K. et al. Life expectancy in marine mammals is unrelated to telomere length but is associated with body size. Front. Genet. 12, 737860 (2021).
Dantzer, B. & Fletcher, Q. E. Telomeres shorten more slowly in slow-aging wild animals than in fast-aging ones. Exp. Gerontol. 71, 38–47 (2015).
Haussmann, M. F. et al. Telomeres shorten more slowly in long-lived birds and mammals than in short-lived ones. Proc. Biol. Sci. 270, 1387–1392 (2003).
Peto, R. Epidemiology, multistage models, and short-term mutagenicity tests. Int. J. Epidemiol. 45, 621–637 (2016).
VanderWalde, N. A. et al. Disparities in older adult accrual to cancer trials: analysis from the Alliance for Clinical Trials in Oncology (A151736). J. Geriatr. Oncol. 13, 20–26 (2022).
Ludmir, E. B. et al. Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol. 5, 1769–1773 (2019).
Guarnieri, C. & Von Hoff, D. D. Phase 1 clinical trials in the elderly: enrollment challenges. J. Adv. Pract. Oncol. 11, 494–501 (2020).
Solary, E., Abou-Zeid, N. & Calvo, F. Ageing and cancer: a research gap to fill. Mol. Oncol. 16, 3220–3237 (2022).
Wong, C. H., Siah, K. W. & Lo, A. W. Estimation of clinical trial success rates and related parameters. Biostatistics 20, 273–286 (2019).
Lukow, D. A. et al. Chromosomal instability accelerates the evolution of resistance to anti-cancer therapies. Dev. Cell 56, 2427–2439 (2021).
Ippolito, M. R. et al. Gene copy-number changes and chromosomal instability induced by aneuploidy confer resistance to chemotherapy. Dev. Cell 56, 2440–2454 (2021).
Maggiorani, D. et al. Senescence drives immunotherapy resistance by inducing an immunosuppressive tumor microenvironment. Nat. Commun. 15, 2435 (2024).
D’Ordine, A. M., Jogl, G. & Sedivy, J. M. Identification and characterization of small molecule inhibitors of the LINE-1 retrotransposon endonuclease. Nat. Commun. 15, 3883 (2024).
Brankiewicz-Kopcinska, W., Kallingal, A., Krzemieniecki, R. & Baginski, M. Targeting shelterin proteins for cancer therapy. Drug Discov. Today 29, 104056 (2024).
Acknowledgements
We thank A. Oppezzo, F. Rossiello, C. Tripodo and A. Bertotti for critically reading the manuscript and M. Cabrini for figure suggestions. L.A.T. is a PhD student within the European School of Molecular Medicine and is supported by an AIRC fellowship for Italy (28211). F.d’A.d.F.’s laboratory is supported by an ERC advanced grant (TELORNAGING, 835103); ERC POC (TELOVACCINE, 101113229); AIRC-IG (30471); AIRC-IG (21762); AIRC 5x1000 (21091); Telethon (GMR23T2007); Progetti di Ricerca di Interesse Nazionale (2020CXFL4T); Progetti di Ricerca di Interesse Nazionale (2022R7LH5T); AriSLA (DDR&ALS FG_24_2020); POR FESR InterSLA (DSB.AD004.294); the Fondazione Regionale per la Ricerca Biomedica (Regione Lombardia) (EJPRD19-206 PROGERIA, GA 825575); Next Generation EU, in the context of the National Recovery and Resilience Plan, Investment PE8 Project Age-It and Investment CN3 National Center for Gene Therapy and Drugs based on RNA Technology.
Author information
Authors and Affiliations
Contributions
All authors conceived, structured, wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
F.d’A.d.F. is a founder and investor in TAG Therapeutics, a company dedicated to treating telomere pathologies and age-related disorders. L.A.T. declares no competing interests.
Peer review
Peer review information
Nature Aging thanks George Garinis, James DeGregori, and Daniel Munoz-Espin for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trastus, L.A., d’Adda di Fagagna, F. The complex interplay between aging and cancer. Nat Aging 5, 350–365 (2025). https://doi.org/10.1038/s43587-025-00827-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43587-025-00827-z
This article is cited by
-
Global epidemiological trend of cancer incidence and death in adults aged 60 years and older: a systematic analysis of data from GLOBOCAN 2022 and GBD2021
BMC Geriatrics (2025)
-
Unravelling the genetics and epigenetics of the ageing tumour microenvironment in cancer
Nature Reviews Cancer (2025)
-
Reprogramming the GRHL2−CDK19 axis by gene therapy alleviates prostate aging
Nature Aging (2025)
-
Using RNA therapeutics to promote healthy aging
Nature Aging (2025)
-
Effects of aging on anticancer therapy in murine cancer models
Cancer and Metastasis Reviews (2025)