Abstract
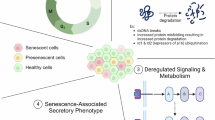
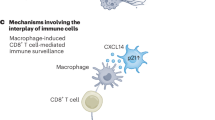
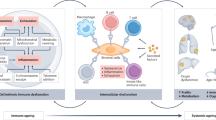
The classical role of adaptive immunity as a protector against external threats has expanded to include its functions in cancer surveillance, tissue repair and regeneration, and, more recently, it has emerged as a regulator of the aging process. In this Perspective, we discuss the mechanisms by which the deterioration of adaptive immunity contributes to inflammaging, cellular senescence and age-associated pathologies. We propose that age-related changes in lymphocytes contribute to aging through two distinct mechanisms. First, adaptive immune function worsens with age, impairing immunosurveillance of damaged or senescent cells and diminishing tissue regenerative potential, thereby indirectly disrupting tissue homeostasis. This disruption is particularly important in the gut, where maintaining tissue and microbiota homeostasis is crucial for overall health during aging. Second, adaptive immune cells often acquire pro-inflammatory and autoaggressive phenotypes with age, directly driving tissue damage, promoting senescence and exacerbating inflammaging. Finally, we explore the therapeutic potential of strategies aimed at enhancing the protective functions of lymphocytes or modulating their pathogenic phenotypes to promote healthy aging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Flajnik, M. F. & Kasahara, M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat. Rev. Genet. 11, 47–59 (2010).
Chi, H., Pepper, M. & Thomas, P. G. Principles and therapeutic applications of adaptive immunity. Cell 187, 2052–2078 (2024).
Farber, D. L., Netea, M. G., Radbruch, A., Rajewsky, K. & Zinkernagel, R. M. Immunological memory: lessons from the past and a look to the future. Nat. Rev. Immunol. 16, 124–128 (2016).
Mittelbrunn, M. & Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 22, 687–698 (2021).
Carpenter, R. S. & Maryanovich, M. Systemic and local regulation of hematopoietic homeostasis in health and disease. Nat. Cardiovasc. Res. 3, 651–665 (2024).
Goronzy, J. J. & Weyand, C. M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 19, 573–583 (2019).
de Mol, J., Kuiper, J., Tsiantoulas, D. & Foks, A. C. The dynamics of B cell aging in health and disease. Front. Immunol. 12, 733566 (2021).
Elyahu, Y. et al. Aging promotes reorganization of the CD4 T cell landscape toward extreme regulatory and effector phenotypes. Sci. Adv. 5, eaaw8330 (2019).
Smit, V. et al. Single-cell profiling reveals age-associated immunity in atherosclerosis. Cardiovasc. Res. 119, 2508–2521 (2023).
Mogilenko, D. A., Shchukina, I. & Artyomov, M. N. Immune ageing at single-cell resolution. Nat. Rev. Immunol. 22, 484–498 (2022).
Zhang, Z. et al. A panoramic view of cell population dynamics in mammalian aging. Science 387, eadn3949 (2025).
Luo, Y. et al. Single-cell genomics identifies distinct B1 cell developmental pathways and reveals aging-related changes in the B-cell receptor repertoire. Cell Biosci. 12, 57 (2022).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254 (2000).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Sayed, N. et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat. Aging 1, 598–615 (2021).
Carrasco, E. et al. The role of T cells in age-related diseases. Nat. Rev. Immunol. 22, 97–111 (2022).
Ma, S., Wang, C., Mao, X. & Hao, Y. B cell dysfunction associated with aging and autoimmune disease. Front. Immunol. 10, 318 (2019).
Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021).
Rafii, P. et al. Constitutive activation of gp130 in T cells results in senescence and premature aging. J. Immunol. 210, 1641–1652 (2023).
Wang, L. et al. Excessive apoptosis of Rip1‐deficient T cells leads to premature aging. EMBO Rep. 24, e57925 (2023).
Desdín-Micó, G. et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368, 1371–1376 (2020).
Huang, W., Hickson, L. T. J., Eirin, A., Kirkland, J. L. & Lerman, L. O. Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 18, 611–627 (2022).
He, S. & Sharpless, N. E. Senescence in health and disease. Cell 169, 1000–1011 (2017).
Sturmlechner, I. et al. p21 produces a bioactive secretome that places stressed cells under immunosurveillance. Science 374, eabb3420 (2021).
Marin, I. et al. Cellular senescence is immunogenic and promotes antitumor immunity. Cancer Discov. 13, 410–431 (2023).
Suda, M. et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat. Aging 1, 1117–1126 (2021).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Yang, D. et al. NKG2D-CAR T cells eliminate senescent cells in aged mice and nonhuman primates. Sci. Transl. Med. 15, eadd1951 (2023).
Pereira, B. I. et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat. Commun. 10, 2387 (2019).
Ovadya, Y. et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 9, 5435 (2018).
Hasegawa, T. et al. Cytotoxic CD4+ T cells eliminate senescent cells by targeting cytomegalovirus antigen. Cell 186, 1417–1431 (2023).
Majewska, J. & Krizhanovsky, V. Immune surveillance of senescent cells in aging and disease. Nat. Aging https://doi.org/10.1038/s43587-025-00910-5 (2025).
Wang, Y. et al. Integrating single-cell RNA and T cell/B cell receptor sequencing with mass cytometry reveals dynamic trajectories of human peripheral immune cells from birth to old age. Nat. Immunol. 26, 308–322 (2025).
Guo, Z. et al. DCAF1 regulates Treg senescence via the ROS axis during immunological aging. J. Clin. Invest. 130, 5893–5908 (2020).
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Dikiy, S. & Rudensky, A. Y. Principles of regulatory T cell function. Immunity 56, 240–255 (2023).
Soto-Heredero, G. et al. KLRG1 identifies regulatory T cells with mitochondrial alterations that accumulate with aging. Nat. Aging 5, 799–815 (2025).
Zhou, L. et al. Centenarians alleviate inflammaging by changing the ratio and secretory phenotypes of T helper 17 and regulatory T cells. Front. Pharmacol. 13, 877709 (2022).
Bozec, A. & Zaiss, M. M. T regulatory cells in bone remodelling. Curr. Osteoporos. Rep. 15, 121–125 (2017).
Xia, N. et al. A unique population of regulatory T cells in heart potentiates cardiac protection from myocardial infarction. Circulation 142, 1956–1973 (2020).
Ding, C. et al. A Treg-specific long noncoding RNA maintains immune–metabolic homeostasis in aging liver. Nat. Aging 3, 813–828 (2023).
Kuswanto, W. et al. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity 44, 355–367 (2016).
Sousa, N. S. et al. The immune landscape of murine skeletal muscle regeneration and aging. Cell Rep. 43, 114975 (2024).
de la Fuente, A. G. et al. Ageing impairs the regenerative capacity of regulatory T cells in mouse central nervous system remyelination. Nat. Commun. 15, 1870 (2024).
Underhill, D. M., Gordon, S., Imhof, B. A., Núñez, G., & Bousso, P. Élie Metchnikoff (1845–1916): celebrating 100 years of cellular immunology and beyond. Nat. Rev. Immunol. 16, 651–656 (2016).
Ghosh, T. S., Shanahan, F. & O’Toole, P. W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 19, 565–584 (2022).
Rera, M., Clark, R. I. & Walker, D. W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl Acad. Sci. USA 109, 21528–21533 (2012).
Huus, K. E., Petersen, C. & Finlay, B. B. Diversity and dynamism of IgA−microbiota interactions. Nat. Rev. Immunol. 21, 514–525 (2021).
Sage, P. T., Tan, C. L., Freeman, G. J., Haigis, M. & Sharpe, A. H. Defective TFH cell function and increased TFR cells contribute to defective antibody production in aging. Cell Rep. 12, 163–171 (2015).
Conway, J. et al. Age-related loss of intestinal barrier integrity plays an integral role in thymic involution and T cell ageing. Aging Cell 24, e14401 (2024).
Širvinskas, D. et al. Single-cell atlas of the aging mouse colon. iScience 25, 104202 (2022).
Kjhn, R., Löhler, I., Rennick, D., Rajewsky, K. & Moiler, W. Interleukin-LO-deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993).
Neumann, C. et al. c-Maf-dependent Treg cell control of intestinal TH17 cells and IgA establishes host–microbiota homeostasis. Nat. Immunol. 20, 471–481 (2019).
Omrani, O. et al. IFNγ–Stat1 axis drives aging-associated loss of intestinal tissue homeostasis and regeneration. Nat. Commun. 14, 6109 (2023).
Fransen, F. et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front. Immunol. 8, 1385 (2017).
Stebegg, M. et al. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat. Commun. 10, 2443 (2019).
Kawamoto, S. et al. Bacterial induction of B cell senescence promotes age-related changes in the gut microbiota. Nat. Cell Biol. 25, 865–876 (2023).
Yang, H. et al. Gut microbial-derived phenylacetylglutamine accelerates host cellular senescence. Nat. Aging 5, 401–418 (2025).
Yang, W. & Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 18, 866–877 (2021).
Lee, J. L. & Linterman, M. A. Mechanisms underpinning poor antibody responses to vaccines in ageing. Immunol. Lett. 241, 1–14 (2022).
Frasca, D. & Blomberg, B. B. Aging induces B cell defects and decreased antibody responses to influenza infection and vaccination. Immun. Ageing 17, 37 (2020).
Ma, S. et al. Spatial transcriptomic landscape unveils immunoglobin-associated senescence as a hallmark of aging. Cell 187, 7025–7044 (2024).
Yu, L. et al. IgG is an aging factor that drives adipose tissue fibrosis and metabolic decline. Cell Metab. 36, 793–807 (2024).
Gudelj, I., Lauc, G. & Pezer, M. Immunoglobulin G glycosylation in aging and diseases. Cell. Immunol. 333, 65–79 (2018).
Plomp, R. et al. Subclass-specific IgG glycosylation is associated with markers of inflammation and metabolic health. Sci. Rep. 7, 12325 (2017).
Giron, L. B. et al. Immunoglobulin G N-glycan markers of accelerated biological aging during chronic HIV infection. Nat. Commun. 15, 3035 (2024).
Streng, B. M. M. et al. IgG1 glycosylation highlights premature aging in Down syndrome. Aging Cell 23, e14167 (2024).
Mogilenko, D. A. et al. Comprehensive profiling of an aging immune system reveals clonal GZMK+ CD8+ T cells as conserved hallmark of inflammaging. Immunity 54, 99–115 (2021).
Frasca, D. et al. B cells with a senescent-associated secretory phenotype accumulate in the adipose tissue of individuals with obesity. Int. J. Mol. Sci. 22, 1839 (2021).
Cancro, M. P. Age-associated B cells. Annu. Rev. Immunol. 38, 315–340 (2020).
Bharath, L. P. et al. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 32, 44–55 (2020).
Baixauli, F. et al. Mitochondrial respiration controls lysosomal function during inflammatory T cell responses. Cell Metab. 22, 485–498 (2015).
Terekhova, M. et al. Single-cell atlas of healthy human blood unveils age-related loss of NKG2C+GZMB−CD8+ memory T cells and accumulation of type 2 memory T cells. Immunity 56, 2836–2854 (2023).
Callender, L. A. et al. Human CD8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell 17, e12675 (2018).
Lan, F. et al. GZMK-expressing CD8+ T cells promote recurrent airway inflammatory diseases. Nature 638, 490–498 (2025).
Guo, C.-L. et al. Granzyme K+CD8+ T cells interact with fibroblasts to promote neutrophilic inflammation in nasal polyps. Nat. Commun. 15, 10413 (2024).
Wang, Z. et al. Pairing of single-cell RNA analysis and T cell antigen receptor profiling indicates breakdown of T cell tolerance checkpoints in atherosclerosis. Nat. Cardiovasc. Res. 2, 290–306 (2023).
Chen, X. et al. Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. Nature 615, 668–677 (2023).
Sulzer, D. et al. T cells of Parkinson’s disease patients recognize α-synuclein peptides. Nature 546, 656–661 (2017).
Mittal, K. et al. CD4 T cells induce a subset of MHCII-expressing microglia that attenuates Alzheimer pathology. iScience 16, 298–311 (2019).
Su, W. et al. CXCR6 orchestrates brain CD8+ T cell residency and limits mouse Alzheimer’s disease pathology. Nat. Immunol. 24, 1735–1747 (2023).
Kedia, S. et al. T cell-mediated microglial activation triggers myelin pathology in a mouse model of amyloidosis. Nat. Neurosci. 27, 1468–1474 (2024).
Panwar, A. et al. Antigen-specific age-related memory CD8 T cells induce and track Alzheimer’s-like neurodegeneration. Proc. Natl Acad. Sci. USA 121, e2401420121 (2024).
Morales-Nebreda, L. et al. Aging imparts cell-autonomous dysfunction to regulatory T cells during recovery from influenza pneumonia. JCI Insight 6, e141690 (2021).
Nishiyama, T. et al. Mechanisms of age-related Treg dysfunction in an arthritic environment. Clin. Immunol. 266, 110337 (2024).
Butcher, M. J. et al. Atherosclerosis-driven Treg plasticity results in formation of a dysfunctional subset of plastic IFNγ+ TH1/Tregs. Circ. Res. 119, 1190–1203 (2016).
Bapat, S. P. et al. Depletion of fat Tregs prevents age-associated insulin resistance. Nature 528, 137–141 (2015).
Ratliff, M., Alter, S., Frasca, D., Blomberg, B. B. & Riley, R. L. In senescence, age-associated B cells secrete TNFα and inhibit survival of B-cell precursors. Aging Cell 12, 303–311 (2013).
Camell, C. D. et al. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab. 30, 1024–1039 (2019).
Rubtsov, A. V. et al. CD11c-expressing B cells are located at the T cell/B cell border in spleen and are potent APCs. J. Immunol. 195, 71–79 (2015).
Li, K. et al. B cells from old mice induce the generation of inflammatory T cells through metabolic pathways. Mech. Ageing Dev. 209, 111742 (2023).
Khan, S. et al. B cells promote T cell immunosenescence and mammalian aging parameters. Preprint at bioRxiv https://doi.org/10.1101/2023.09.12.556363 (2023).
Goto, M. et al. Age-associated CD4+ T cells with B cell-promoting functions are regulated by ZEB2 in autoimmunity. Sci. Immunol. 9, eadk1643 (2024).
Carey, A. et al. Age-associated accumulation of B cells promotes macrophage inflammation and inhibits lipolysis in adipose tissue during sepsis. Cell Rep. 43, 113967 (2024).
Kaya, T. et al. CD8+ T cells induce interferon-responsive oligodendrocytes and microglia in white matter aging. Nat. Neurosci. 25, 1446–1457 (2022).
Gabandé-Rodríguez, E. et al. Cytotoxic CD4+ T cells in the bone marrow compromise healthy ageing by enhancing granulopoiesis. Preprint at bioRxiv https://doi.org/10.1101/2024.01.26.577360 (2024).
Hao, Y., O’Neill, P., Naradikian, M. S., Scholz, J. L. & Cancro, M. P. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 118, 1294–1304 (2011).
Rubtsov, A. V. et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood 118, 1305–1315 (2011).
Pereira, B. I. et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol. 21, 684–694 (2020).
Dudek, M. et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature 592, 444–449 (2021).
Takeuchi, A. & Saito, T. CD4 CTL, a cytotoxic subset of CD4+ T cells, their differentiation and function. Front. Immunol. 8, 194 (2017).
Mucida, D. et al. Transcriptional reprogramming of mature CD4+ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat. Immunol. 14, 281–289 (2013).
Hashimoto, K. et al. Single-cell transcriptomics reveals expansion of cytotoxic CD4 T cells in supercentenarians. Proc. Natl Acad. Sci. USA 116, 24242–24251 (2019).
Oh, D. Y. & Fong, L. Cytotoxic CD4+ T cells in cancer: expanding the immune effector toolbox. Immunity 54, 2701–2711 (2021).
Wang, P. et al. Single-cell transcriptome and TCR profiling reveal activated and expanded T cell populations in Parkinson’s disease. Cell Discov. 7, 52 (2021).
Piehl, N. et al. Cerebrospinal fluid immune dysregulation during healthy brain aging and cognitive impairment. Cell 185, 5028–5039 (2022).
Di Francesco, A. et al. Dietary restriction impacts health and lifespan of genetically diverse mice. Nature 634, 684–692 (2024).
Messaoudi, I. et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc. Natl Acad. Sci. USA 103, 19448–19453 (2006).
Spadaro, O. et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science 375, 671–677 (2022).
Eikawa, S. et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc. Natl Acad. Sci. USA 112, 1809–1814 (2015).
Yang, J. et al. The effect of metformin on senescence of T lymphocytes. Immun. Ageing 20, 73 (2023).
Mannick, J. B. & Lamming, D. W. Targeting the biology of aging with mTOR inhibitors. Nat. Aging 3, 642–660 (2023).
Chen, C., Liu, Y., Liu, Y. & Zheng, P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci. Signal. 2, ra75 (2009).
Ando, S. et al. mTOR regulates T cell exhaustion and PD-1-targeted immunotherapy response during chronic viral infection. J. Clin. Invest. 133, e160025 (2023).
Mannick, J. B. et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 6, 268ra179 (2014).
Girotra, M. et al. Induction of mitochondrial recycling reverts age-associated decline of the hematopoietic and immune systems. Nat. Aging 3, 1057–1066 (2023).
Zhao, J. et al. ATM is a key driver of NF-κB-dependent DNA-damage-induced senescence, stem cell dysfunction and aging. Aging 12, 4688–4710 (2020).
Arora, S. et al. Invariant natural killer T cells coordinate removal of senescent cells. Med 2, 938–950 (2021).
Ma, S. et al. Heterochronic parabiosis induces stem cell revitalization and systemic rejuvenation across aged tissues. Cell Stem Cell 29, 990–1005 (2022).
Ho, T. T. et al. Aged hematopoietic stem cells are refractory to bloodborne systemic rejuvenation interventions. J. Exp. Med. 218, e20210223 (2021).
Davies, J. S., Thompson, H. L., Pulko, V., Padilla Torres, J. & Nikolich-Žugich, J. Role of cell-intrinsic and environmental age-related changes in altered maintenance of murine T cells in lymphoid organs. J. Gerontol. A Biol. Sci. Med. Sci. 73, 1018–1026 (2018).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Dahlquist, K. J. V. et al. PD1 blockade improves survival and CD8+ cytotoxic capacity, without increasing inflammation, during normal microbial experience in old mice. Nat. Aging 4, 915–925 (2024).
Hamilton, S. E. et al. New insights into the immune system using dirty mice. J. Immunol. 205, 3–11 (2020).
Ahuja, S. K. et al. Immune resilience despite inflammatory stress promotes longevity and favorable health outcomes including resistance to infection. Nat. Commun. 14, 3286 (2023).
Ross, J. B. et al. Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity. Nature 628, 162–170 (2024).
Delemarre, E. M. et al. Autologous stem cell transplantation aids autoimmune patients by functional renewal and TCR diversification of regulatory T cells. Blood 127, 91–101 (2016).
Shim, H. S. et al. TERT activation targets DNA methylation and multiple aging hallmarks. Cell 187, 4030–4042 (2024).
Karagiannis, P., Iriguchi, S. & Kaneko, S. Reprogramming away from the exhausted T cell state. Semin. Immunol. 28, 35–44 (2016).
Lanna, A. et al. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nat. Cell Biol. 24, 1461–1474 (2022).
Baker, D. J., Arany, Z., Baur, J. A., Epstein, J. A. & June, C. H. CAR T therapy beyond cancer: the evolution of a living drug. Nature 619, 707–715 (2023).
Deng, Y. et al. Targeting senescent cells with NKG2D-CAR T cells. Cell Death Discov. 10, 217 (2024).
Amor, C. et al. Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nat. Aging 4, 336–349 (2024).
Eskiocak, O. et al. Senolytic CAR T cells reverse aging-associated defects in intestinal regeneration and fitness. Preprint at bioRxiv https://doi.org/10.1101/2024.03.19.585779 (2024).
Ming, X. et al. A chimeric peptide promotes immune surveillance of senescent cells in injury, fibrosis, tumorigenesis and aging. Nat. Aging 5, 28–47 (2025).
Widjaja, A. A. et al. Inhibition of IL-11 signalling extends mammalian healthspan and lifespan. Nature 632, 157–165 (2024).
Lainé, A. et al. Regulatory T cells promote cancer immune-escape through integrin αvβ8-mediated TGF-β activation. Nat. Commun. 12, 6228 (2021).
Acknowledgements
Funded by the European Union (M.M.). Views and opinions expressed are, however, those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. Neither the European Union nor the granting authority can be held responsible for them. This work was supported by the European Research Council (ERC-2021-CoG 101044248-Let T Be) (M.M.) and Spanish Ministerio de Ciencia e Innovación (PID2022-141169OB-I00) grants (M.M.), NIH grant R21AG087361 (M.J.Y.), a Nelson Family Transplant Innovation Award (M.J.Y.), pilot projects from the New York Nutrition and Obesity Research Center and the CALERIE study (M.J.Y.). S.D.-P. was supported by a PIPF grant (PIPF-2022/SAL-GL-25208) from the Comunidad de Madrid (Spain).
Author information
Authors and Affiliations
Contributions
S.D.-P., M.J.Y. and M.M. contributed to the preparation of the Perspective.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Alon Monsonego, Jessica Lancaster, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Delgado-Pulido, S., Yousefzadeh, M.J. & Mittelbrunn, M. Aging reshapes the adaptive immune system from healer to saboteur. Nat Aging 5, 1393–1403 (2025). https://doi.org/10.1038/s43587-025-00906-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43587-025-00906-1
This article is cited by
-
Targeting a master inflammatory switch in the aging cochlea attenuates sensory decline
The Egyptian Journal of Otolaryngology (2026)
-
Rejuvenating the aging gut by targeting senescence
Nature Aging (2026)
-
A natural compound revitalizes the aging human immune system
Nature Aging (2025)