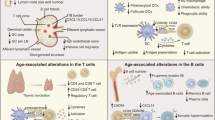
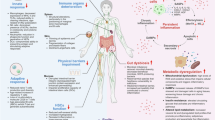
Abstract
Cellular aging of the immune system, commonly referred to as ‘immunosenescence’, drives a substantial decline in vaccine efficacy among older adults, who are already typically at a higher risk of reduced infection control. Therefore, preventive medicine requires novel strategies to improve vaccination in older adults, particularly by finding ways to mitigate immunosenescence and chronic inflammation. Here, we review how technical innovations, such as increased antigen amounts, improved adjuvants and mRNA-based and universal vaccines, can complement traditional methods of improving vaccination success in older adults. Furthermore, we discuss emerging clinical evidence suggesting that geroscience interventions, such as mTOR inhibition and caloric restriction, can enhance vaccine outcomes in older adults, potentially by targeting molecular aspects of immunosenescence and systemic characteristics of aging, including metabolic changes in the blood and chronic inflammation. Ultimately, we propose that integrating geroscience with tailored immunization could attenuate the effects of immune aging on vaccination efficacy in older populations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kallestrup-Lamb, M., Marin, A. O. K., Menon, S. & Søgaard, J. Aging populations and expenditures on health. J. Econ. Ageing 29, 100518 (2024).
Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021).
Pinti, M. et al. Aging of the immune system: focus on inflammation and vaccination. Eur. J. Immunol. 46, 2286–2301 (2016).
Borgoni, S., Kudryashova, K. S., Burka, K. & de Magalhães, J. P. Targeting immune dysfunction in aging. Ageing Res. Rev. 70, 101410 (2021).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Newnham, D. M. & Hamilton, S. J. C. Sensitivity of the cough reflex in young and elderly subjects. Age Ageing 26, 185–188 (1997).
Sagiv, M. S. in Exercise Cardiopulmonary Function in Cardiac Patients (ed. Sagiv, M. S.) 171–194 (Springer, 2012).
Brandenberger, C. & Mühlfeld, C. Mechanisms of lung aging. Cell Tissue Res. 367, 469–480 (2017).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Kirkwood, K. L. Inflammaging. Immunol. Invest. 47, 770–773 (2018).
Li, X. et al. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 8, 239 (2023).
Davidson, S. et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 21, 704–717 (2021).
Liu, Z. et al. Immunosenescence: molecular mechanisms and diseases. Signal Transduct. Target. Ther. 8, 200 (2023).
Desdín-Micó, G. et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368, 1371–1376 (2020).
Waldman, S. A. & Terzic, A. Health care evolves from reactive to proactive. Clin. Pharmacol. Ther. 105, 10–13 (2019).
Pollard, A. J. & Bijker, E. M. A guide to vaccinology: from basic principles to new developments. Nat. Rev. Immunol. 21, 83–100 (2021).
Pangilinan, A. R., Brangman, S. A., Gravenstein, S., Schmader, K. & Kuchel, G. A. Vaccinations in older adults: optimization, strategies, and latest guidelines. J. Am. Geriatr. Soc. 73, 20–28 (2025).
Jefferson, T. et al. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 366, 1165–1174 (2005).
Osterholm, M. T., Kelley, N. S., Sommer, A. & Belongia, E. A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 12, 36–44 (2012).
Lee, J. L. & Linterman, M. A. Mechanisms underpinning poor antibody responses to vaccines in ageing. Immunol. Lett. 241, 1–14 (2022).
Kwetkat, A. & Heppner, H. J. Comorbidities in the elderly and their possible influence on vaccine response. Interdiscip. Top. Gerontol. Geriatr. 43, 73–85 (2020).
Bianchi, F. P. & Tafuri, S. Vaccination of elderly people affected by chronic diseases: a challenge for public health. Vaccines (Basel) 10, 641 (2022).
Painter, S. D., Ovsyannikova, I. G. & Poland, G. A. The weight of obesity on the human immune response to vaccination. Vaccine 33, 4422–4429 (2015).
Kennedy, B. K. et al. Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-021-00411-4 (2022).
Lee, D. J. W., Kuerec, A. H. & Maier, A. B. Targeting ageing with rapamycin and its derivatives in humans: a systematic review. Lancet Healthy Longev. 5, e152–e162 (2024).
Di Francesco, A. et al. Dietary restriction impacts health and lifespan of genetically diverse mice. Nature 634, 684–692 (2024).
COVID-19 Forecasting Team.Variation in the COVID-19 infection–fatality ratio by age, time, and geography during the pre-vaccine era: a systematic analysis. Lancet 399, 1469–1488 (2022).
Bonanad, C. et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J. Am. Med. Dir. Assoc. 21, 915–918 (2020).
Guesneau, C. et al. Risk factors associated with 30-day mortality in older patients with influenza. J. Clin. Med. 10, 3521 (2021).
Yang, S. et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci. Rep. 6, 27251 (2016).
Jílková, E., Vejvalková, P., Stiborová, I., Skorkovský, J. & Král, V. Serological response to tick-borne encephalitis (TBE) vaccination in the elderly—results from an observational study. Expert Opin. Biol. Ther. 9, 797–803 (2009).
Antonelli Incalzi, R. et al. Influenza vaccination for elderly, vulnerable and high-risk subjects: a narrative review and expert opinion. Intern. Emerg. Med. 19, 619–640 (2024).
Collier, D. A. et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 596, 417–422 (2021).
López-Fauqued, M. et al. Safety profile of the adjuvanted recombinant zoster vaccine: pooled analysis of two large randomised phase 3 trials. Vaccine 37, 2482–2493 (2019).
Cunningham, A. L. et al. Immune responses to a recombinant glycoprotein E herpes zoster vaccine in adults aged 50 years or older. J. Infect. Dis. 217, 1750–1760 (2018).
Dagnew, A. F. et al. The adjuvanted recombinant zoster vaccine in adults aged ≥65 years previously vaccinated with a live-attenuated herpes zoster vaccine. J. Infect. Dis. 224, 1139–1146 (2021).
Lal, H. et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 372, 2087–2096 (2015).
Cunningham, A. L. et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N. Engl. J. Med. 375, 1019–1032 (2016).
Zhao, T. et al. Vaccine adjuvants: mechanisms and platforms. Signal Transduct. Target. Ther. 8, 283 (2023).
Yu, J., Peng, J. & Chi, H. Systems immunology: integrating multi-omics data to infer regulatory networks and hidden drivers of immunity. Curr. Opin. Syst. Biol. 15, 19–29 (2019).
Davis, M. M., Tato, C. M. & Furman, D. Systems immunology: just getting started. Nat. Immunol. 18, 725–732 (2017).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020).
Weichhart, T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology 64, 127–134 (2018).
Shimobayashi, M. & Hall, M. N. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 15, 155–162 (2014).
Jin, J. et al. Activation of mTORC1 at late endosomes misdirects T cell fate decision in older individuals. Sci. Immunol. 6, eabg0791 (2021).
Teissier, T., Boulanger, E. & Cox, L. S. Interconnections between inflammageing and immunosenescence during ageing. Cells 11, 359 (2022).
Han, S., Georgiev, P., Ringel, A. E., Sharpe, A. H. & Haigis, M. C. Age-associated remodeling of T cell immunity and metabolism. Cell Metab. 35, 36–55 (2023).
Slaets, H., Veeningen, N., de Keizer, P. L. J., Hellings, N. & Hendrix, S. Are immunosenescent T cells really senescent? Aging Cell 23, e14300 (2024).
Zhou, D., Borsa, M. & Simon, A. K. Hallmarks and detection techniques of cellular senescence and cellular ageing in immune cells. Aging Cell 20, e13316 (2021).
Effros, R. B. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine 25, 599–604 (2007).
Panwar, V. et al. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 8, 375 (2023).
Cornu, M., Albert, V. & Hall, M. N. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev. 23, 53–62 (2013).
Alsaleh, G. et al. Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses. eLife 9, e57950 (2020).
Puleston, D. J. et al. Autophagy is a critical regulator of memory CD8+ T cell formation. eLife 3, e03706 (2014).
Escrig-Larena, J. I., Delgado-Pulido, S. & Mittelbrunn, M. Mitochondria during T cell aging. Semin. Immunol. 69, 101808 (2023).
Salminen, A. & Kaarniranta, K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 11, 230–241 (2012).
Foretz, M., Guigas, B. & Viollet, B. Metformin: update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 19, 460–476 (2023).
Feldman, N., Rotter-Maskowitz, A. & Okun, E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res. Rev. 24, 29–39 (2015).
Zanini, G. et al. Mitochondrial DNA as inflammatory DAMP: a warning of an aging immune system? Biochem. Soc. Trans. 51, 735–745 (2023).
Widjaja, A. A. et al. Inhibition of IL-11 signalling extends mammalian healthspan and lifespan. Nature 632, 157–165 (2024).
Ross, J. B. et al. Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity. Nature 628, 162–170 (2024).
Geiger, H., de Haan, G. & Florian, M. C. The ageing haematopoietic stem cell compartment. Nat. Rev. Immunol. 13, 376–389 (2013).
Mortensen, M. et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J. Exp. Med. 208, 455–467 (2011).
Chougnet, C. A. et al. Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J. Immunol. 195, 2624–2632 (2015).
Wong, C. & Goldstein, D. R. Impact of aging on antigen presentation cell function of dendritic cells. Curr. Opin. Immunol. 25, 535–541 (2013).
Tabula Muris Consortium.A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595 (2020).
Kurioka, A. & Klenerman, P. Aging unconventionally: γδ T cells, iNKT cells, and MAIT cells in aging. Semin. Immunol. 69, 101816 (2023).
Thomas, R., Wang, W. & Su, D.-M. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun. Ageing 17, 2 (2020).
Kousa, A. I. et al. Age-related epithelial defects limit thymic function and regeneration. Nat. Immunol. 25, 1593–1606 (2024).
Goronzy, J. J., Fang, F., Cavanagh, M. M., Qi, Q. & Weyand, C. M. Naive T cell maintenance and function in human aging. J. Immunol. 194, 4073–4080 (2015).
Aiello, A. et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 10, 2247 (2019).
Muller, G. C. et al. The inverted CD4:CD8 ratio is associated with gender-related changes in oxidative stress during aging. Cell. Immunol. 296, 149–154 (2015).
Yam-Puc, J. C. et al. Age-associated B cells predict impaired humoral immunity after COVID-19 vaccination in patients receiving immune checkpoint blockade. Nat. Commun. 14, 3292 (2023).
Frasca, D., Diaz, A., Romero, M. & Blomberg, B. B. The generation of memory B cells is maintained, but the antibody response is not, in the elderly after repeated influenza immunizations. Vaccine 34, 2834–2840 (2016).
Shaw, A. C., Joshi, S., Greenwood, H., Panda, A. & Lord, J. M. Aging of the innate immune system. Curr. Opin. Immunol. 22, 507–513 (2010).
Mittelbrunn, M. & Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 22, 687–698 (2021).
Frasca, D., Diaz, A., Romero, M., Garcia, D. & Blomberg, B. B. B cell immunosenescence. Annu. Rev. Cell Dev. Biol. 36, 551–574 (2020).
Bulati, M., Caruso, C. & Colonna-Romano, G. From lymphopoiesis to plasma cells differentiation, the age-related modifications of B cell compartment are influenced by “inflamm-ageing”. Ageing Res. Rev. 36, 125–136 (2017).
Wrona, M. V., Ghosh, R., Coll, K., Chun, C. & Yousefzadeh, M. J. The 3 I’s of immunity and aging: immunosenescence, inflammaging, and immune resilience. Front. Aging 5, 1490302 (2024).
Cakala-Jakimowicz, M., Kolodziej-Wojnar, P. & Puzianowska-Kuznicka, M. Aging-related cellular, structural and functional changes in the lymph nodes: a significant component of immunosenescence? An overview. Cells 10, 3148 (2021).
Krishnamurty, A. T. & Turley, S. J. Lymph node stromal cells: cartographers of the immune system. Nat. Immunol. 21, 369–380 (2020).
Sonar, S. A., Bhat, R., Coplen, C. P. & Nikolich-Zugich, J. The age-related changes in lymph node stromal cells underlie defects in peripheral T cell maintenance and immune function decline in old mice. J. Immunol. 210, 239.16 (2023).
Shankwitz, K. et al. Compromised steady-state germinal center activity with age in nonhuman primates. Aging Cell 19, e13087 (2020).
Foster, W. S., Marcial-Juárez, E. & Linterman, M. A. The cellular factors that impair the germinal center in advanced age. J. Immunol. 214, 862–871 (2025).
Ng, Y. Y. & Tay, A. Exploring lymph node stroma ageing: immune implications and future directions. Aging Cell 24, e70000 (2025).
Lancaster, J. N. Aging of lymphoid stromal architecture impacts immune responses. Semin. Immunol. 70, 101817 (2023).
Plotkin, S. A. & Boppana, S. B. Vaccination against the human cytomegalovirus. Vaccine 37, 7437–7442 (2019).
Klenerman, P. & Oxenius, A. T cell responses to cytomegalovirus. Nat. Rev. Immunol. 16, 367–377 (2016).
van den Berg, S. P. H., Warmink, K., Borghans, J. A. M., Knol, M. J. & van Baarle, D. Effect of latent cytomegalovirus infection on the antibody response to influenza vaccination: a systematic review and meta-analysis. Med. Microbiol. Immunol. 208, 305–321 (2019).
Breznik, J. A. et al. Cytomegalovirus seropositivity in older adults changes the T cell repertoire but does not prevent antibody or cellular responses to SARS-CoV-2 vaccination. J. Immunol. 209, 1892–1905 (2022).
Hu, X. et al. Human cytomegalovirus mRNA-1647 vaccine candidate elicits potent and broad neutralization and higher antibody-dependent cellular cytotoxicity responses than the gB/MF59 vaccine. J. Infect. Dis. 230, 455–466 (2024).
Klenerman, P. & Zinkernagel, R. M. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature 394, 482–485 (1998).
Kim, H., Webster, R. G. & Webby, R. J. Influenza virus: dealing with a drifting and shifting pathogen. Viral Immunol. 31, 174–183 (2018).
Tortorici, M. A. et al. Persistent immune imprinting occurs after vaccination with the COVID-19 XBB.1.5 mRNA booster in humans. Immunity 57, 904–911 (2024).
Ding, X. et al. Original antigenic sin: a potential double-edged effect for vaccine improvement. Biomed. Pharmacother. 178, 117187 (2024).
Hancock, K. et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 361, 1945–1952 (2009).
Weinberger, B. Vaccines for the elderly: current use and future challenges. Immun. Ageing 15, 3 (2018).
Wiggins, K. B., Smith, M. A. & Schultz-Cherry, S. The nature of immune responses to influenza vaccination in high-risk populations. Viruses 13, 1109 (2021).
Sang, D. et al. Prolonged sleep deprivation induces a cytokine-storm-like syndrome in mammals. Cell 186, 5500–5516 (2023).
Al-Rashed, F. et al. Impact of sleep deprivation on monocyte subclasses and function. J. Immunol. https://doi.org/10.1093/jimmun/vkae016 (2025).
Besedovsky, L., Lange, T. & Born, J. Sleep and immune function. Pflugers Arch. 463, 121–137 (2012).
Cabrera, Á. J. R. Zinc, aging, and immunosenescence: an overview. Pathobiol. Aging Age Relat. Dis. https://doi.org/10.3402/pba.v5.25592 (2015).
Chillon, T. S. et al. Serum free zinc is associated with vaccination response to SARS-CoV-2. Front. Immunol. 13, 906551 (2022).
Kaml, M. et al. Booster vaccination in the elderly: their success depends on the vaccine type applied earlier in life as well as on pre-vaccination antibody titers. Vaccine 24, 6808–6811 (2006).
Park, H. J. et al. Comparing frequency of booster vaccination to prevent severe COVID-19 by risk group in the United States. Nat. Commun. 15, 1883 (2024).
Lee, J. K. H., Lam, G. K. L., Yin, J. K., Loiacono, M. M. & Samson, S. I. High-dose influenza vaccine in older adults by age and seasonal characteristics: systematic review and meta-analysis update. Vaccine X 14, 100327 (2023).
Comber, L. et al. Systematic review of the efficacy, effectiveness and safety of high-dose seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in individuals ≥18 years of age. Rev. Med. Virol. 33, e2330 (2023).
Reed, S. G., Orr, M. T. & Fox, C. B. Key roles of adjuvants in modern vaccines. Nat. Med. 19, 1597–1608 (2013).
Ko, E.-J. & Kang, S.-M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccin. Immunother. 14, 3041–3045 (2018).
Reisinger, K. S., Holmes, S. J., Pedotti, P., Arora, A. K. & Lattanzi, M. A dose-ranging study of MF59®-adjuvanted and non-adjuvanted A/H1N1 pandemic influenza vaccine in young to middle-aged and older adult populations to assess safety, immunogenicity, and antibody persistence one year after vaccination. Hum. Vaccin. Immunother. 10, 2395–2407 (2014).
Rodrigues, L. P. et al. Hallmarks of aging and immunosenescence: connecting the dots. Cytokine Growth Factor Rev. 59, 9–21 (2021).
Cadar, A. N., Martin, D. E. & Bartley, J. M. Targeting the hallmarks of aging to improve influenza vaccine responses in older adults. Immun. Ageing 20, 23 (2023).
Hofer, S. J., Carmona-Gutierrez, D., Mueller, M. I. & Madeo, F. The ups and downs of caloric restriction and fasting: from molecular effects to clinical application. EMBO Mol. Med. https://doi.org/10.15252/emmm.202114418 (2022).
Chung, K. W. & Chung, H. Y. The effects of calorie restriction on autophagy: role on aging intervention. Nutrients 11, 2923 (2019).
González, O. A., Tobia, C., Ebersole, J. L. & Novak, M. J. Caloric restriction and chronic inflammatory diseases. Oral Dis. 18, 16–31 (2012).
Kökten, T. et al. Calorie restriction as a new treatment of inflammatory diseases. Adv. Nutr. 12, 1558–1570 (2021).
Effros, R. B., Walford, R. L., Weindruch, R. & Mitcheltree, C. Influences of dietary restriction on immunity to influenza in aged mice. J. Gerontol. 46, B142–B147 (1991).
Janssen, H. et al. Monocytes re-enter the bone marrow during fasting and alter the host response to infection. Immunity 56, 783–796 (2023).
Jordan, S. et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 178, 1102–1114 (2019).
Collins, N. et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 178, 1088–1101 (2019).
Goldberg, E. L. & Dixit, V. D. Bone marrow: an immunometabolic refuge during energy depletion. Cell Metab. 30, 621–623 (2019).
Chen, H. et al. Intermittent fasting promotes type 3 innate lymphoid cells secreting IL-22 contributing to the beigeing of white adipose tissue. eLife 12, RP91060 (2024).
Ahmed, T. et al. Calorie restriction enhances T-cell-mediated immune response in adult overweight men and women. J. Gerontol. A Biol. Sci. Med. Sci. 64, 1107–1113 (2009).
Han, S.-C. et al. Intermittent fasting modulates immune response by generating Tregs via TGF-β dependent mechanisms in obese mice with allergic contact dermatitis. Biomol. Ther. (Seoul) 32, 136–145 (2024).
He, Z., Xu, H., Li, C., Yang, H. & Mao, Y. Intermittent fasting and immunomodulatory effects: a systematic review. Front. Nutr. 10, 1048230 (2023).
Horne, B. D. et al. Association of periodic fasting with lower severity of COVID-19 outcomes in the SARS-CoV-2 prevaccine era: an observational cohort from the INSPIRE registry. BMJ Nutr. Prev. Health 5, 145–153 (2022).
Kim, Y. et al. Time-restricted feeding reduces monocyte production by controlling hematopoietic stem and progenitor cells in the bone marrow during obesity. Front. Immunol. 13, 1054875 (2022).
Qian, J. et al. Innate immune remodeling by short-term intensive fasting. Aging Cell 20, e13507 (2021).
Chen, Y. et al. Time-restricted eating reveals a “younger” immune system and reshapes the intestinal microbiome in human. Redox Biol. 78, 103422 (2024).
Spadaro, O. et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science 375, 671–677 (2022).
Meydani, S. N. et al. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging (Albany NY) 8, 1416–1431 (2016).
Tizazu, A. M. Fasting and calorie restriction modulate age-associated immunosenescence and inflammaging. Aging Med. (Milton) 7, 499–509 (2024).
Ma, R.-X. A detective story of intermittent fasting effect on immunity. Immunology 173, 227–247 (2024).
Hofer, S. J. & Madeo, F. Sex- and timing-specific effects of fasting and caloric restriction. Cell Metab. 35, 1091–1093 (2023).
Ma, D., Li, S., Molusky, M. M. & Lin, J. D. Circadian autophagy rhythm: a link between clock and metabolism? Trends Endocrinol. Metab. 23, 319–325 (2012).
Otasowie, C. O., Tanner, R., Ray, D. W., Austyn, J. M. & Coventry, B. J. Chronovaccination: harnessing circadian rhythms to optimize immunisation strategies. Front. Immunol. 13, 977525 (2022).
Cervantes-Silva, M. P. et al. The circadian clock influences T cell responses to vaccination by regulating dendritic cell antigen processing. Nat. Commun. 13, 7217 (2022).
Ulgherait, M. et al. Circadian autophagy drives iTRF-mediated longevity. Nature 598, 353–358 (2021).
Phillips, A. C., Gallagher, S., Carroll, D. & Drayson, M. Preliminary evidence that morning vaccination is associated with an enhanced antibody response in men. Psychophysiology 45, 663–666 (2008).
Long, J. E. et al. Morning vaccination enhances antibody response over afternoon vaccination: a cluster-randomised trial. Vaccine 34, 2679–2685 (2016).
de Bree, L. C. J. et al. Circadian rhythm influences induction of trained immunity by BCG vaccination. J. Clin. Invest. https://doi.org/10.1172/JCI133934 (2020).
Zhang, H. et al. Time of day influences immune response to an inactivated vaccine against SARS-CoV-2. Cell Res. 31, 1215–1217 (2021).
Dumont, F. J. & Su, Q. Mechanism of action of the immunosuppressant rapamycin. Life Sci. 58, 373–395 (1996).
Robida-Stubbs, S. et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 15, 713–724 (2012).
Mannick, J. B. et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 6, 268ra179 (2014).
Kraig, E. et al. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: immunological, physical performance, and cognitive effects. Exp. Gerontol. 105, 53–69 (2018).
Diniz, M. O. et al. NK cells limit therapeutic vaccine-induced CD8+T cell immunity in a PD-L1-dependent manner. Sci. Transl. Med. 14, eabi4670 (2022).
Rahman, S. A. et al. PD-1 blockade and vaccination provide therapeutic benefit against SIV by inducing broad and functional CD8+ T cells in lymphoid tissue. Sci. Immunol. 6, eabh3034 (2021).
Mannick, J. B. et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med. 10, eaaq1564 (2018).
Mannick, J. B. et al. Targeting the biology of ageing with mTOR inhibitors to improve immune function in older adults: phase 2b and phase 3 randomised trials. Lancet Healthy Longev. 2, e250–e262 (2021).
Hörbelt, T. et al. Dose-dependent acute effects of everolimus administration on immunological, neuroendocrine and psychological parameters in healthy men. Clin. Transl. Sci. 13, 1251–1259 (2020).
Thoreen, C. C. et al. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485, 109–113 (2012).
Chamoto, K., Zhang, B., Tajima, M., Honjo, T. & Fagarasan, S. Spermidine—an old molecule with a new age-defying immune function. Trends Cell Biol. 34, 363–370 (2024).
Schroeder, S. et al. Dietary spermidine improves cognitive function. Cell Rep. 35, 108985 (2021).
Hofer, S. J. et al. Spermidine-induced hypusination preserves mitochondrial and cognitive function during aging. Autophagy 17, 2037–2039 (2021).
Al-Habsi, M. et al. Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. Science 378, eabj3510 (2022).
Liu, R. et al. Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Radic. Biol. Med. 161, 339–350 (2020).
Hofer, S. J. et al. Mechanisms of spermidine-induced autophagy and geroprotection. Nat. Aging https://doi.org/10.1038/s43587-022-00322-9 (2022).
Eisenberg, T. et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 22, 1428–1438 (2016).
Hofer, S. J. et al. Spermidine is essential for fasting-mediated autophagy and longevity. Nat. Cell Biol. https://doi.org/10.1038/s41556-024-01468-x (2024).
Hofer, S. J. et al. A surge in endogenous spermidine is essential for rapamycin-induced autophagy and longevity. Autophagy 20, 2824–2826 (2024).
Choi, Y. H. & Park, H. Y. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J. Biomed. Sci. 19, 31 (2012).
Puleston, D. J. et al. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell 184, 4186–4202 (2021).
Wagner, A. et al. Metabolic modeling of single Th17 cells reveals regulators of autoimmunity. Cell 184, 4168–4185 (2021).
Alsaleh, G. et al. Spermidine mitigates immune cell senescence, enhances autophagy, and boosts vaccine responses in healthy older adults. Preprint at ResearchSquare https://doi.org/10.21203/rs.3.rs-5686388/v1 (2025).
Madeo, F., Carmona-Gutierrez, D., Hofer, S. J. & Kroemer, G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 29, 592–610 (2019).
Böhme, J. et al. Metformin enhances anti-mycobacterial responses by educating CD8+ T-cell immunometabolic circuits. Nat. Commun. 11, 5225 (2020).
Frasca, D., Diaz, A., Romero, M. & Blomberg, B. B. Metformin enhances B cell function and antibody responses of elderly individuals with type-2 diabetes mellitus. Front. Aging 2, 715981 (2021).
Martin, D. E. et al. The effect of metformin on influenza vaccine responses in nondiabetic older adults: a pilot trial. Immun. Ageing 20, 18 (2023).
Yen, F.-S., Wang, S.-I., Lin, S.-Y. & Wei, J. C.-C. Metformin use before COVID-19 vaccination and the risks of COVID-19 incidence, medical utilization, and all-cause mortality in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 200, 110692 (2023).
Chaib, S., Tchkonia, T. & Kirkland, J. L. Cellular senescence and senolytics: the path to the clinic. Nat. Med. 28, 1556–1568 (2022).
Camell, C. D. et al. Senolytics reduce coronavirus-related mortality in old mice. Science 373, eabe4832 (2021).
Lee, S. et al. Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature 599, 283–289 (2021).
Torrance, B. L. et al. Senolytic treatment with dasatinib and quercetin does not improve overall influenza responses in aged mice. Front. Aging 4, 1212750 (2023).
Luna, A. et al. Senolytic treatment attenuates immune cell infiltration without improving IAV outcomes in aged mice. Aging Cell 24, e14437 (2025).
Henson, D. A., Kohut, M. L., Nieman, D. C., Heinz, S. A. & Yin, F. Quercetin supplementation does not enhance antibody responses to influenza vaccination. FASEB J. 24, 723.5 (2010).
Du, P. Y., Gandhi, A., Bawa, M. & Gromala, J. The ageing immune system as a potential target of senolytics. Oxf. Open Immunol. 4, iqad004 (2023).
Walzik, D., Wences Chirino, T. Y., Zimmer, P. & Joisten, N. Molecular insights of exercise therapy in disease prevention and treatment. Signal Transduct. Target. Ther. 9, 138 (2024).
Zhou, X.-H., Luo, Y.-X. & Yao, X.-Q. Exercise-driven cellular autophagy: a bridge to systematic wellness. J. Adv. Res. https://doi.org/10.1016/j.jare.2024.12.036 (2025).
Hallam, J., Jones, T., Alley, J. & Kohut, M. L. Exercise after influenza or COVID-19 vaccination increases serum antibody without an increase in side effects. Brain Behav. Immun. 102, 1–10 (2022).
Batatinha, H. et al. Recent COVID-19 vaccination has minimal effects on the physiological responses to graded exercise in physically active healthy people. J. Appl. Physiol. (1985) 132, 275–282 (2022).
Smith, K. A. et al. COVID-19 vaccination produces exercise-responsive SARS-CoV-2 specific T-cells regardless of infection history. J. Sport Health Sci. 13, 99–107 (2024).
Baker, F. L. et al. Exercise mobilizes diverse antigen specific T-cells and elevates neutralizing antibodies in humans with natural immunity to SARS CoV-2. Brain Behav. Immun. Health 28, 100600 (2023).
Zuckerman, J. N. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ 321, 1237–1238 (2000).
Elzayat, M. T., Markofski, M. M., Simpson, R. J., Laughlin, M. & LaVoy, E. C. No effect of acute eccentric resistance exercise on immune responses to influenza vaccination in older adults: a randomized control trial. Front. Physiol. 12, 713183 (2021).
Bohn-Goldbaum, E. et al. Acute exercise decreases vaccine reactions following influenza vaccination among older adults. Brain Behav. Immun. Health 1, 100009 (2019).
Abdellatif, M., Sedej, S. & Kroemer, G. NAD+ metabolism in cardiac health, aging, and disease. Circulation 144, 1795–1817 (2021).
Fang, J. et al. NAD+ metabolism-based immunoregulation and therapeutic potential. Cell Biosci. 13, 81 (2023).
Fang, E. F. et al. NAD+ in aging: molecular mechanisms and translational implications. Trends Mol. Med. 23, 899–916 (2017).
Zhang, H. et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443 (2016).
Mitchell, S. J. et al. Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Cell Metab. 27, 667–676 (2018).
Gombart, A. F., Pierre, A. & Maggini, S. A review of micronutrients and the immune system—working in harmony to reduce the risk of infection. Nutrients 12, 236 (2020).
Carr, A. C. & Gombart, A. F. Multi-level immune support by vitamins C and D during the SARS-CoV-2 pandemic. Nutrients 14, 689 (2022).
Mora, J. R., Iwata, M. & von Andrian, U. H. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 8, 685–698 (2008).
Wu, M., He, M. & Kang, Y. Vitamin C supplementation improved the efficacy of foot-and-mouth disease vaccine. Food Agric. Immunol. 29, 470–483 (2018).
Sindi, N. Evaluation of the efficacy of vitamin C on the immune response after rabies virus vaccine in BALB/c mice. Eur. Rev. Med. Pharmacol. Sci. 27, 1808–1815 (2023).
Sadarangani, S. P., Whitaker, J. A. & Poland, G. A. “Let there be light”: the role of vitamin D in the immune response to vaccines. Expert Rev. Vaccines 14, 1427–1440 (2015).
Cesur, F., Atasever, Z. & Özoran, Y. Impact of vitamin D3 supplementation on COVID-19 vaccine response and immunoglobulin G antibodies in deficient women: a randomized controlled trial. Vaccine 41, 2860–2867 (2023).
Goncalves-Mendes, N. et al. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front. Immunol. 10, 65 (2019).
Lord, J. M. The effect of aging of the immune system on vaccination responses. Hum. Vaccin. Immunother. 9, 1364–1367 (2013).
Goetz, L. H. & Schork, N. J. Personalized medicine: motivation, challenges, and progress. Fertil. Steril. 109, 952–963 (2018).
Fourati, S. et al. Pan-vaccine analysis reveals innate immune endotypes predictive of antibody responses to vaccination. Nat. Immunol. 23, 1777–1787 (2022).
Tsang, J. S. et al. Improving vaccine-induced immunity: can baseline predict outcome? Trends Immunol. 41, 457–465 (2020).
Bechman, K., Russell, M. D. & Galloway, J. B. Predicting COVID-19 vaccination response in populations who are immunosuppressed. Lancet Rheumatol. 5, e431–e432 (2023).
Hagan, T. et al. Transcriptional atlas of the human immune response to 13 vaccines reveals a common predictor of vaccine-induced antibody responses. Nat. Immunol. 23, 1788–1798 (2022).
Cortese, M. et al. System vaccinology analysis of predictors and mechanisms of antibody response durability to multiple vaccines in humans. Nat. Immunol. 26, 116–130 (2025).
Pearce, F. A. et al. Antibody prevalence after three or more COVID-19 vaccine doses in individuals who are immunosuppressed in the UK: a cross-sectional study from MELODY. Lancet Rheumatol. 5, e461–e473 (2023).
Kumar, S. et al. Systemic dysregulation and molecular insights into poor influenza vaccine response in the aging population. Sci. Adv. 10, eadq7006 (2024).
Li, Y., Wu, X., Fang, D. & Luo, Y. Informing immunotherapy with multi-omics driven machine learning. NPJ Digit. Med. 7, 67 (2024).
Wu, Y. & Xie, L. AI-driven multi-omics integration for multi-scale predictive modeling of genotype–environment–phenotype relationships. Comput. Struct. Biotechnol. J. 27, 265–277 (2025).
Martínez de Toda, I., Vida, C., Díaz-Del Cerro, E. & De la Fuente, M. The immunity clock. J. Gerontol. A Biol. Sci. Med. Sci. 76, 1939–1945 (2021).
Sayed, N. et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat. Aging 1, 598–615 (2021).
Kalyakulina, A. et al. Small immunological clocks identified by deep learning and gradient boosting. Front. Immunol. 14, 1177611 (2023).
Ye, T. et al. Inhaled SARS-CoV-2 vaccine for single-dose dry powder aerosol immunization. Nature 624, 630–638 (2023).
Kim, Y. C., Jarrahian, C., Zehrung, D., Mitragotri, S. & Prausnitz, M. R. Delivery systems for intradermal vaccination. Curr. Top. Microbiol. Immunol. 351, 77–112 (2012).
Kashem, S. W., Haniffa, M. & Kaplan, D. H. Antigen-presenting cells in the skin. Annu. Rev. Immunol. 35, 469–499 (2017).
Egunsola, O. et al. Immunogenicity and safety of reduced-dose intradermal vs intramuscular influenza vaccines: a systematic review and meta-analysis. JAMA Netw. Open 4, e2035693 (2021).
Holland, D. et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J. Infect. Dis. 198, 650–658 (2008).
Hofer, S. J., Davinelli, S., Bergmann, M., Scapagnini, G. & Madeo, F. Caloric restriction mimetics in nutrition and clinical trials. Front. Nutr. 8, 717343 (2021).
Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20, 817–838 (2021).
Rijkers, G. T. et al. Antigen presentation of mRNA-based and virus-vectored SARS-CoV-2 vaccines. Vaccines (Basel) 9, 848 (2021).
Xie, C., Yao, R. & Xia, X. The advances of adjuvants in mRNA vaccines. NPJ Vaccines 8, 162 (2023).
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021).
Amor, C. et al. Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nat. Aging 4, 336–349 (2024).
Denton, A. E. et al. Targeting TLR4 during vaccination boosts MAdCAM-1+ lymphoid stromal cell activation and promotes the aged germinal center response. Sci. Immunol. 7, eabk0018 (2022).
Wang, G. et al. Universal subunit vaccine protects against multiple SARS-CoV-2 variants and SARS-CoV. NPJ Vaccines 9, 133 (2024).
Wang, W.-C., Sayedahmed, E. E., Sambhara, S. & Mittal, S. K. Progress towards the development of a universal influenza vaccine. Viruses 14, 1684 (2022).
Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 19, 383–397 (2019).
Nachbagauer, R. et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 27, 106–114 (2021).
Bravi, B. Development and use of machine learning algorithms in vaccine target selection. NPJ Vaccines 9, 15 (2024).
Olawade, D. B. et al. Leveraging artificial intelligence in vaccine development: a narrative review. J. Microbiol. Methods 224, 106998 (2024).
Abdelmohsen, K. et al. Identification of senescent cell subpopulations by CITE-seq analysis. Aging Cell 23, e14297 (2024).
Bouazzaoui, A. & Abdellatif, A. A. H. Vaccine delivery systems and administration routes: advanced biotechnological techniques to improve the immunization efficacy. Vaccine X 19, 100500 (2024).
Lavelle, E. C. & Ward, R. W. Mucosal vaccines—fortifying the frontiers. Nat. Rev. Immunol. 22, 236–250 (2022).
Lee, V. Y., Booy, R., Skinner, S. R., Fong, J. & Edwards, K. M. The effect of exercise on local and systemic adverse reactions after vaccinations—outcomes of two randomized controlled trials. Vaccine 36, 6995–7002 (2018).
de Boer, S. E. et al. Enhanced humoral immune response after COVID-19 vaccination in elderly kidney transplant recipients on everolimus versus mycophenolate mofetil-containing immunosuppressive regimens. Transplantation 106, 1615–1621 (2022).
de Boer, S. E. et al. Rationale and design of the OPTIMIZE trial: OPen label multicenter randomized trial comparing standard IMmunosuppression with tacrolimus and mycophenolate mofetil with a low exposure tacrolimus regimen In combination with everolimus in de novo renal transplantation in Elderly patients. BMC Nephrol. 22, 208 (2021).
Tunbridge, M. et al. Rapamycin and inulin for booster vaccine response stimulation (RIVASTIM)–rapamycin: study protocol for a randomised, controlled trial of immunosuppression modification with rapamycin to improve SARS-CoV-2 vaccine response in kidney transplant recipients. Trials 23, 780 (2022).
Withers, H. G. et al. mTOR inhibition modulates vaccine-induced immune responses to generate memory T cells in patients with solid tumors. J. Immunother. Cancer 13, e010408 (2025).
Ji, N. et al. Rapamycin enhances BCG-specific γδ T cells during intravesical BCG therapy for non-muscle invasive bladder cancer: a randomized, double-blind study. J. Immunother. Cancer 9, e001941 (2021).
Acknowledgements
P.K. is funded by the Wellcome Trust (222426/Z/21/Z), a Medical Research Council IMMPROVE Proactive Vaccinology grant and Cancer Research UK (DRCNPG-Nov22/100005). A.K.S. is supported by the Wellcome Trust Fund 220784/Z/20/Z and the Helmholtz Distinguished Professorship.
Author information
Authors and Affiliations
Contributions
S.J.H. and A.K.S. conceptualized the review. All authors contributed to writing and editing the paper.
Corresponding author
Ethics declarations
Competing interests
A.K.S. has received consultancy fees from TLL The Longevity Labs GmbH, Oxford Healthspan and Kalin Health. P.K. has received consulting fees from UCB, Biomunex, AstraZeneca and Infinitopes. S.J.H. and S.R. declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Jorg Goronzy, Sean Leng and the other anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hofer, S.J., Rapp, S., Klenerman, P. et al. Understanding and improving vaccine efficacy in older adults. Nat Aging 5, 1455–1470 (2025). https://doi.org/10.1038/s43587-025-00939-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43587-025-00939-6