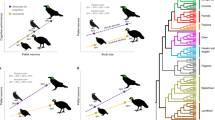
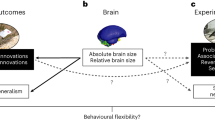
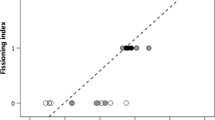
Abstract
Avian species are one of the most diverse and adaptable groups of animals: there are far more species of birds than of mammals, and they occupy a broad range of habitats. Birds and mammals split from a common ancestor over 300 million years ago. Yet certain bird species can perform complex mental tasks, including numerical problems, at levels similar to — and in some cases surpassing — primates, including great apes. Birds thus offer a privileged perspective on the cognitive functions underlying numerical abilities and their evolution. Moreover, birds provide excellent models for studying the ontogenetic development and neural mechanisms underlying numerical computations. In this Review, we provide a comprehensive picture of the contribution of avian studies to understanding numerical cognition, including behavioural laboratory studies, field studies and neurobiological investigations. We also critically examine the methodologies, interpretations and limitations of selected key studies. By synthesizing current knowledge and situating it within the broader field of cognitive research, we highlight the importance of a comparative perspective in understanding the role of evolutionary convergence in the emergence of cognitive functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Koehler, O. Vom Erlernen unbenannter Anzahlen bei Vögeln. Naturwissenschaften 29, 201–218 (1941).
Dawson, B. V. Counting in jackdaws. Behaviour 18, 229–238 (1961).
Pastore, N. Number sense and ‘counting’ ability in the canary. Z. Tierpsychol. 18, 561–573 (1961).
Koehler, O. Ability of birds to count. Nature 168, 373–375 (1951).
Marold, E. Versuche an Wellensittichen zur Frage des “Zähl”-Vermögens. Z. Tierpsychol. 3, 170–223 (1939).
Pfungst, O. Das Pferd des Herrn von Osten: Der kluge Hans. Ein Beitrag zur experimentellen Tier- und Menschen-Psychologie (J. A. Barth, 1907).
Boysen, S. T. & Capaldi, E. J. The Development of Numerical Competence: Animal and Human Models (Psychology Press, 1993).
Haun, D. B. M., Jordan, F. M., Vallortigara, G. & Clayton, N. S. Origins of spatial, temporal and numerical cognition: insights from comparative psychology. Trends Cogn. Sci. 14, 552–560 (2010).
Güntürkün, O., Pusch, R. & Rose, J. Why birds are smart. Trends Cogn. Sci. 28, 197–209 (2024).
Swenson, L. C. One versus two discrimination by whitenecked ravens (Corvus cryptoleucus) with non-number dimensions varied. Anim. Behav. 18, 454–460 (1970).
Smirnova, A. A., Lazareva, O. F. & Zorina, Z. A. Use of number by crows: investigation by matching and oddity learning. J. Exp. Anal. Behav. 73, 163–176 (2000).
Emmerton, J. & Renner, J. C. Scalar effects in the visual discrimination of numerosity by pigeons. Learn. Behav. 34, 176–192 (2006).
Rugani, R., Vallortigara, G. & Regolin, L. The use of proportion by young domestic chicks (Gallus gallus). Anim. Cogn. 18, 605–616 (2015). This article shows that domestic chicks can learn to select stimuli according to the proportion of red and green.
Rugani, R., McCrink, K., de Hevia, M.-D., Vallortigara, G. & Regolin, L. Ratio abstraction over discrete magnitudes by newly hatched domestic chicks (Gallus gallus). Sci. Rep. 6, 30114 (2016).
Roberts, W. A., MacDonald, H. & Lo, K. H. Pigeons play the percentages: computation of probability in a bird. Anim. Cogn. 21, 575–581 (2018).
Garland, A., Low, J. & Burns, K. C. Large quantity discrimination by North Island robins (Petroica longipes). Anim. Cogn. 15, 1129–1140 (2012). This article uses an interesting approach to test food quantity discrimination in the wild.
Lyon, B. E. Egg recognition and counting reduce costs of avian conspecific brood parasitism. Nature 422, 495–499 (2003).
Harper, D. G. C. Competitive foraging in mallards: ‘ideal free’ ducks. Anim. Behav. 30, 575–584 (1982). This article shows how different (numerical) factors need to be taken into account when birds make foraging decisions.
Scarf, D., Hayne, H. & Colombo, M. Pigeons on par with primates in numerical competence. Science 334, 1664–1664 (2011).
Güntürkün, O. & Bugnyar, T. Cognition without cortex. Trends Cogn. Sci. 20, 291–303 (2016).
Dugas-Ford, J. & Ragsdale, C. W. Levels of homology and the problem of neocortex. Annu. Rev. Neurosci. 38, 351–368 (2015).
Bai, Y. et al. A review of brain-inspired cognition and navigation technology for mobile robots. Cyborg Bionic Syst. 5, 0128 (2024).
Koehler, O. “Zähl”-Versuche an einem Kolkraben und Vergleichsversuche an Menschen. Z. Tierpsychol. 5, 575–712 (1943).
Spelke, E. S. Principles of object perception. Cogn. Sci. 14, 29–56 (1990).
Feigenson, L., Carey, S. & Hauser, M. The representations underlying infants’ choice of more: object files versus analog magnitudes. Psychol. Sci. 13, 150–156 (2002).
Cantlon, J. F. & Brannon, E. M. Shared system for ordering small and large numbers in monkeys and humans. Psychol. Sci. 17, 401–406 (2006).
Cordes, S., Gelman, R., Gallistel, C. R. & Whalen, J. Variability signatures distinguish verbal from nonverbal counting for both large and small numbers. Psychon. Bull. Rev. 8, 698–707 (2001).
Gallistel, C. R. & Gelman, R. Preverbal and verbal counting and computation. Cognition 44, 43–74 (1992).
Vallortigara, G., Chiandetti, C., Rugani, R., Sovrano, V. A. & Regolin, L. Animal cognition. Wiley Interdisc. Rev. Cogn. Sci. 1, 882–893 (2010).
Davis, H. & Pérusse, R. Numerical competence in animals: definitional issues, current evidence, and a new research agenda. Behav. Brain Sci. 11, 561–579 (1988). This article provides basic definitions and concepts for the field of numerical cognition.
Rugani, R., Castiello, U., Priftis, K., Spoto, A. & Sartori, L. What is a number? The interplay between number and continuous magnitudes. Behav. Brain Sci. 40, e187 (2017).
Bogale, B. A., Kamata, N., Mioko, K. & Sugita, S. Quantity discrimination in jungle crows, Corvus macrorhynchos. Anim. Behav. 82, 635–641 (2011).
Gebuis, T., Cohen Kadosh, R. & Gevers, W. Sensory-integration system rather than approximate number system underlies numerosity processing: a critical review. Acta Psychol. 171, 17–35 (2016).
Wu, P., Zhu, J., He, Q., Wang, Z. & Shi, L. Visual numerical cognition in pigeons: conformity to the Weber–Fechner law. Anim. Cogn. 28, 39 (2025).
McCrink, K. & Wynn, K. Ratio abstraction by 6-month-old infants. Psychol. Sci. 18, 740–745 (2007).
Coburn, C. A. & Yerkes, R. M. A study of the behavior of the crow Corvus americanus Aud. by the multiple choice method. J. Anim. Behav. 5, 75–114 (1915).
Davis, H. & Bradford, S. Counting behavior by rats in a simulated natural environment. Ethology 73, 265–280 (1986).
Suzuki, K. & Kobayashi, T. Numerical competence in rats (Rattus norvegicus): Davis and Bradford (1986) extended. J. Comp. Psychol. 114, 73–85 (2000).
Chittka, L. & Geiger, K. Can honey bees count landmarks? Anim. Behav. 49, 159–164 (1995).
Dacke, M. & Srinivasan, M. V. Evidence for counting in insects. Anim. Cogn. 11, 683–689 (2008).
Rugani, R., Kelly, D. M., Szelest, I., Regolin, L. & Vallortigara, G. Is it only humans that count from left to right? Biol. Lett. 6, 290–292 (2010).
Rugani, R., Regolin, L. & Vallortigara, G. Rudimental numerical competence in 5-day-old domestic chicks (Gallus gallus): identification of ordinal position. J. Exp. Psychol. Anim. Behav. Process. 33, 21–31 (2007). This study showed that birds (domestic chicks) can identify objects based on their ordinal position within a sequence under various conditions.
Rugani, R., Vallortigara, G., Vallini, B. & Regolin, L. Asymmetrical number-space mapping in the avian brain. Neurobiol. Learn. Mem. 95, 231–238 (2010).
Vámos, T. I. F., Tello-Ramos, M. C., Hurly, T. A. & Healy, S. D. Numerical ordinality in a wild nectarivore. Proc. R. Soc. B 287, 20201269 (2020). This article provides an example of an ordinal task (identifying an object according to its ordinal position within a sequence) in the wild using artificial flowers.
Brannon, E. M. & Terrace, H. S. Ordering of the numerosities 1 to 9 by monkeys. Science 282, 746–749 (1998).
Wynn, K. Addition and subtraction by human infants. Nature 358, 749–750 (1992).
Garland, A. & Low, J. Addition and subtraction in wild New Zealand robins. Behav. Process. 109, 103–110 (2014). This article uses an interesting approach to test for mental arithmetic in the wild.
Pepperberg, I. M. Grey parrot (Psittacus erithacus) numerical abilities: addition and further experiments on a zero-like concept. J. Comp. Psychol. 120, 1–11 (2006).
Liao, D. A., Brecht, K. F., Veit, L. & Nieder, A. Crows “count” the number of self-generated vocalizations. Science 384, 874–877 (2024).
Moyer, R. S. & Landauer, T. K. Time required for judgements of numerical inequality. Nature 215, 1519–1520 (1967).
Rugani, R., Regolin, L. & Vallortigara, G. Imprinted numbers: newborn chicks’ sensitivity to number vs. continuous extent of objects they have been reared with. Dev. Sci. 13, 790–797 (2010).
Rugani, R., Fontanari, L., Simoni, E., Regolin, L. & Vallortigara, G. Arithmetic in newborn chicks. Proc. R. Soc. B 276, 2451–2460 (2009).
Nieder, A. The adaptive value of numerical competence. Trends Ecol. Evol. 35, 605–617 (2020).
Hunt, S., Low, J. & Burns, K. C. Adaptive numerical competency in a food-hoarding songbird. Proc. R. Soc. B 275, 2373–2379 (2008).
Guigueno, M. F., Coto, M. A. & Sherry, D. F. Brood-parasitic female cowbirds have better numerical abilities than males on a task resembling nest prospecting behaviour. Biol. Lett. 21, 20240670 (2025).
Krebs, J. R. Colonial nesting and social feeding as strategies for exploiting food resources in the great blue heron (Ardea Herodias). Behaviour 51, 99–134 (1974).
Tornick, J. K., Callahan, E. S. & Gibson, B. M. An investigation of quantity discrimination in Clark’s nutcrackers (Nucifraga columbiana). J. Comp. Psychol. 129, 17–25 (2015).
Kelly, E. M. Counting on your friends: the role of social environment on quantity discrimination. Behav. Process. 128, 9–16 (2016).
Rahman, N. A. A., Fadzly, N., Dzakwan, N. M. & Zulkifli, N. H. The numerical competency of two bird species (Corvus splendens and Acridotheres tristis). Trop. Life Sci. Res. 25, 95–103 (2014).
Gallistel, C. R. The Organization of Learning viii, 648 (MIT Press, 1990).
Hunter, H., Blackburn, G., Ashton, B. J. & Ridley, A. R. Group size affects spontaneous quantity discrimination performance in wild Western Australian magpies (Gymnorhina tibicen dorsalis). Anim. Cogn. 28, 41 (2025).
Elgar, M. A. Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biol. Rev. Camb. Phil. Soc. 64, 13–33 (1989).
Bahr, D. B. & Bekoff, M. Predicting flock vigilance from simple passerine interactions: modelling with cellular automata. Anim. Behav. 58, 831–839 (1999).
Bekoff, M. Vigilance, flock size, and flock geometry: information gathering by western evening grosbeaks (Aves, Fringillidae). Ethology 99, 150–161 (1995).
Li, C., Zhou, L., Li, H. & Jiang, Z. Effects of foraging mode and group pattern on vigilance behavior in water birds: a case study of mallard and black-winged stilt. Belg. J. Zool. 141, 45–54 (2011).
Thompson, N. S. Counting and communication in crows. Commun. Behav. Biol. 31, 223–225 (1968).
Templeton, C. N., Greene, E. & Davis, K. Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308, 1934–1937 (2005).
Seddon, N. & Tobias, J. A. Communal singing in the cooperatively breeding subdesert mesite monias benschi: evidence of numerical assessment? J. Avian Biol. 34, 72–80 (2003).
Stiller, J. et al. Complexity of avian evolution revealed by family-level genomes. Nature 629, 851–860 (2024).
Lambert, M. L., Jacobs, I., Osvath, M. & von Bayern, A. M. P. Birds of a feather? Parrot and corvid cognition compared. Behaviour 156, 505–594 (2019).
Pika, S., Sima, M. J., Blum, C. R., Herrmann, E. & Mundry, R. Ravens parallel great apes in physical and social cognitive skills. Sci. Rep. 10, 20617 (2020).
Sol, D. et al. Neuron numbers link innovativeness with both absolute and relative brain size in birds. Nat. Ecol. Evol. 6, 1381–1389 (2022).
Jarvis, E. D. et al. Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 6, 151–159 (2005).
Butler, A. B., Reiner, A. & Karten, H. J. Evolution of the amniote pallium and the origins of mammalian neocortex. Ann. NY Acad. Sci. 1225, 14–27 (2011).
Karten, H. J. Vertebrate brains and evolutionary connectomics: on the origins of the mammalian ‘neocortex’. Phil. Trans. R. Soc. B 370, 20150060 (2015).
Puelles, L. Current status of the hypothesis of a claustro-insular homolog in sauropsids. Brain. Behav. Evol. 96, 212–241 (2022).
Stacho, M. et al. A cortex-like canonical circuit in the avian forebrain. Science 369, eabc5534 (2020).
Rueda-Alaña, E. et al. Evolutionary convergence of sensory circuits in the pallium of amniotes. Science 387, eadp3411 (2025).
Zaremba, B. et al. Developmental origins and evolution of pallial cell types and structures in birds. Science 387, eadp5182 (2025).
Gattoni, G. & Tosches, M. A. Constrained roads to complex brains. Science 387, 716–717 (2025).
Hecker, N. et al. Enhancer-driven cell type comparison reveals similarities between the mammalian and bird pallium. Science 387, eadp3957 (2025).
Clark, W. J. & Colombo, M. The functional architecture, receptive field characteristics, and representation of objects in the visual network of the pigeon brain. Prog. Neurobiol. 195, 101781 (2020).
Bischof, H.-J. et al. Multiple visual field representations in the visual Wulst of a laterally eyed bird, the zebra finch (Taeniopygia guttata). PLoS ONE 11, e0154927 (2016).
Watanabe, S., Mayer, U. & Bischof, H.-J. Pattern discrimination is affected by entopallial but not by hippocampal lesions in zebra finches. Behav. Brain Res. 190, 201–205 (2008).
Watanabe, S., Mayer, U. & Bischof, H.-J. Visual Wulst analyses “where” and entopallium analyses “what” in the zebra finch visual system. Behav. Brain Res. 222, 51–56 (2011).
Lorenzi, E., Perrino, M. & Vallortigara, G. Numerosities and other magnitudes in the brains: a comparative view. Front. Psychol. 12, 641994 (2021).
Messina, A. et al. Response to change in the number of visual stimuli in zebrafish: a behavioural and molecular study. Sci. Rep. 10, 5769 (2020).
Messina, A. et al. Neurons in the dorso-central division of zebrafish pallium respond to change in visual numerosity. Cereb. Cortex 32, 418–428 (2022).
Kovas, Y. et al. Brain correlates of non-symbolic numerosity estimation in low and high mathematical ability children. PLoS ONE 4, e4587 (2009).
Collins, A. G. E., Ciullo, B., Frank, M. J. & Badre, D. Working memory load strengthens reward prediction errors. J. Neurosci. 37, 4332–4342 (2017).
Lorenzi, E., Perrino, M., Messina, A., Zanon, M. & Vallortigara, G. Innate responses to numerousness reveal neural activation in different brain regions in newly-hatched visually naïve chicks. Heliyon 10, e34162 (2024).
Roitman, J. D., Brannon, E. M. & Platt, M. L. Monotonic coding of numerosity in macaque lateral intraparietal area. PLoS Biol. 5, e208 (2007).
Welford, A. T. The measurement of sensory-motor performance: survey and reappraisal of twelve years’ progress. Ergonomics 3, 189–230 (1960).
Nieder, A. & Merten, K. A labeled-line code for small and large numerosities in the monkey prefrontal cortex. J. Neurosci. 27, 5986–5993 (2007).
Sawamura, H., Shima, K. & Tanji, J. Numerical representation for action in the parietal cortex of the monkey. Nature 415, 918–922 (2002).
Nieder, A. Supramodal numerosity selectivity of neurons in primate prefrontal and posterior parietal cortices. Proc. Natl Acad. Sci. 109, 11860–11865 (2012).
Güntürkün, O. The avian ‘prefrontal cortex’ and cognition. Curr. Opin. Neurobiol. 15, 686–693 (2005).
Güntürkün, O., von Eugen, K., Packheiser, J. & Pusch, R. Avian pallial circuits and cognition: a comparison to mammals. Curr. Opin. Neurobiol. 71, 29–36 (2021).
Ditz, H. M. & Nieder, A. Neurons selective to the number of visual items in the corvid songbird endbrain. Proc. Natl Acad. Sci. 112, 7827–7832 (2015). This article describes the presence of number-sensitive neurons in corvids that, like other bird species, lack a neocortex.
Ditz, H. M. & Nieder, A. Numerosity representations in crows obey the Weber–Fechner law. Proc. R. Soc. B 283, 20160083 (2016).
Wagener, L. & Nieder, A. Categorical representation of abstract spatial magnitudes in the executive telencephalon of crows. Curr. Biol. 33, 2151–2162.e5 (2023).
Kobylkov, D., Mayer, U., Zanon, M. & Vallortigara, G. Number neurons in the nidopallium of young domestic chicks. Proc. Natl Acad. Sci. 119, e2201039119 (2022).
Wagener, L., Loconsole, M., Ditz, H. M. & Nieder, A. Neurons in the endbrain of numerically naive crows spontaneously encode visual numerosity. Curr. Biol. 28, 1090–1094 (2018).
Kirschhock, M. E. & Nieder, A. Number selective sensorimotor neurons in the crow translate perceived numerosity into number of actions. Nat. Commun. 13, 6913 (2022).
Kirschhock, M. E. & Nieder, A. Association neurons in the crow telencephalon link visual signs to numerical values. Proc. Natl Acad. Sci. 120, e2313923120 (2023).
Ditz, H. M., Fechner, J. & Nieder, A. Cell-type specific pallial circuits shape categorical tuning responses in the crow telencephalon. Commun. Biol. 5, 269 (2022).
Diester, I. & Nieder, A. Complementary contributions of prefrontal neuron classes in abstract numerical categorization. J. Neurosci. 28, 7737–7747 (2008).
Reiner, A. Could theropod dinosaurs have evolved to a human level of intelligence? J. Comp. Neurol. 531, 975–1006 (2023).
Tinbergen, N. On aims and methods of ethology. Z. Tierpsychol. 20, 410–433 (1963).
Chiandetti, C. & Vallortigara, G. in Field and Laboratory Methods in Animal Cognition: a Comparative Guide (eds Amici, F. & Bueno-Guerra, N.) 97–118 (Cambridge Univ. Press, 2018).
Gottlieb, G. & Lickliter, R. The various roles of animal models in understanding human development. Soc. Dev. 13, 311–325 (2004).
Lickliter, R. The influence of prenatal experience on behavioral and social development: the benefits and limitations of an animal model. Dev. Psychopathol. 30, 871–880 (2018).
Butterworth, B. The development of arithmetical abilities. J. Child. Psychol. Psychiatry 46, 3–18 (2005).
de Hevia, M. D., Izard, V., Coubart, A., Spelke, E. S. & Streri, A. Representations of space, time, and number in neonates. Proc. Natl Acad. Sci. USA. 111, 4809–4813 (2014).
Rugani, R., Regolin, L. & Vallortigara, G. Discrimination of small numerosities in young chicks. J. Exp. Psychol. Anim. Behav. Process. 34, 388–399 (2008).
Feigenson, L., Carey, S. & Spelke, E. Infants’ discrimination of number vs. continuous extent. Cogn. Psychol. 44, 33–66 (2002).
Rugani, R. Towards numerical cognition’s origin: insights from day-old domestic chicks. Phil. Trans. R. Soc. B 373, 20160509 (2018).
Berger, A., Tzur, G. & Posner, M. I. Infant brains detect arithmetic errors. Proc. Natl Acad. Sci. 103, 12649–12653 (2006).
Christodoulou, J., Lac, A. & Moore, D. S. Babies and math: a meta-analysis of infants’ simple arithmetic competence. Dev. Psychol. 53, 1405–1417 (2017).
Feigenson, L. & Carey, S. Tracking individuals via object-files: evidence from infants’ manual search. Dev. Sci. 6, 568–584 (2003).
Starr, A. B., Libertus, M. E. & Brannon, E. M. Infants show ratio-dependent number discrimination regardless of set size. Infancy https://doi.org/10.1111/infa.12008 (2013).
Spelke, E. S. & Kinzler, K. D. Core knowledge. Dev. Sci. 10, 89–96 (2007).
Feigenson, L., Dehaene, S. & Spelke, E. Core systems of number. Trends Cogn. Sci. 8, 307–314 (2004).
Libertus, M. E. & Brannon, E. M. Stable individual differences in number discrimination in infancy. Dev. Sci. 13, 900–906 (2010).
Scarf, D. & Colombo, M. Knowledge of the ordinal position of list items in pigeons. J. Exp. Psychol. Anim. Behav. Process. 37, 483–487 (2011).
Pepperberg, I. M. Grey parrot numerical competence: a review. Anim. Cogn. 9, 377–391 (2006). This review summarizes the achievements and abilities of one of the most outstanding bird subjects in animal cognition research.
Ujfalussy, D. J., Miklósi, Á., Bugnyar, T. & Kotrschal, K. Role of mental representations in quantity judgments by jackdaws (Corvus monedula). J. Comp. Psychol. 128, 11–20 (2014).
Pepperberg, I. M. & Carey, S. Grey parrot number acquisition: the inference of cardinal value from ordinal position on the numeral list. Cognition 125, 219–232 (2012).
Pepperberg, I. M. & Gordon, J. D. Number comprehension by a grey parrot (Psittacus erithacus), including a zero-like concept. J. Comp. Psychol. 119, 197–209 (2005).
Kirschhock, M. E., Ditz, H. M. & Nieder, A. Behavioral and neuronal representation of numerosity zero in the crow. J. Neurosci. 41, 4889–4896 (2021).
Nieder, A. Absolute numerosity discrimination as a case study in comparative vertebrate intelligence. Front. Psychol. 11, 1843 (2020).
Agrillo, C. in The Oxford Handbook of Numerical Cognition (eds. Kadosh, R. C. & Dowker, A.) 214–236 (Oxford Univ. Press, 2015).
Uller, C. in Computation, Cognition, and Pylyshyn (eds Dedrick, D. & Trick, L.) 219–243 (The MIT Press, 2009).
Meng, W. Editorial: application and research progress of avian models in neuroscience. Front. Mol. Neurosci. 16, 1319308 (2023).
Marino, L. Thinking chickens: a review of cognition, emotion, and behavior in the domestic chicken. Anim. Cogn. 20, 127–147 (2017).
Galton, F. Visualised numerals. Nature 21, 252–256 (1880).
Shaki, S., Fischer, M. H. & Petrusic, W. M. Reading habits for both words and numbers contribute to the SNARC effect. Psychon. Bull. Rev. 16, 328–331 (2009).
Zebian, S. Linkages between number concepts, spatial thinking, and directionality of writing: the SNARC effect and the reverse SNARC effect in English and Arabic monoliterates, biliterates, and illiterate Arabic speakers. J. Cogn. Cult. 5, 165–190 (2005).
Brugger, P. Animal behavior. Chicks with a number sense. Science 347, 477–478 (2015).
Rugani, R., Vallortigara, G., Priftis, K. & Regolin, L. Numerical magnitude, rather than individual bias, explains spatial numerical association in newborn chicks. eLife 9, e54662 (2020).
de Hevia, M. D., Veggiotti, L., Streri, A. & Bonn, C. D. At birth, humans associate ‘few’ with left and ‘many’ with right. Curr. Biol. 27, 3879–3884.e2 (2017).
Di Giorgio, E. et al. A mental number line in human newborns. Dev. Sci. 22, e12801 (2019).
Giurfa, M., Marcout, C., Hilpert, P., Thevenot, C. & Rugani, R. An insect brain organizes numbers on a left-to-right mental number line. Proc. Natl Acad. Sci. 119, e2203584119 (2022).
Beran, M. J., French, K., Smith, T. R. & Parrish, A. E. Limited evidence of number-space mapping in rhesus monkeys (Macaca mulatta) and capuchin monkeys (Sapajus apella). J. Comp. Psychol. 133, 281–293 (2019).
Triki, Z. & Bshary, R. Cleaner fish Labroides dimidiatus discriminate numbers but fail a mental number line test. Anim. Cogn. 21, 99–107 (2018).
Loconsole, M., Regolin, L. & Rugani, R. Asymmetric number–space association leads to more efficient processing of congruent information in domestic chicks. Front. Behav. Neurosci. 17, 1115662 (2023).
Rugani, R., Rosa Salva, O. & Regolin, L. Lateralized mechanisms for encoding of object. Behavioral evidence from an animal model: the domestic chick (Gallus gallus). Front. Psychol. 5, 150 (2014).
Rugani, R., Vallortigara, G., Priftis, K. & Regolin, L. Number-space mapping in the newborn chick resembles humans’ mental number line. Science 347, 534–536 (2015).
Vallortigara, G. Comparative cognition of number and space: the case of geometry and of the mental number line. Phil. Trans. R. Soc. B 373, 20170120 (2018).
Felisatti, A., Laubrock, J., Shaki, S. & Fischer, M. H. A biological foundation for spatial-numerical associations: the brain’s asymmetric frequency tuning. Ann. NY Acad. Sci. 1477, 44–53 (2020).
Cowan, W. M., Adamson, L. & Powell, T. P. An experimental study of the avian visual system. J. Anat. 95, 545–563 (1961).
Andrew, R. J. Neural and Behavioral Plasticity: The Use of the Domestic Chick as a Model (Oxford Univ. Press, 1991).
Mihrshahi, R. The corpus callosum as an evolutionary innovation. J. Exp. Zool. B 306, 8–17 (2006).
Andrew, R. J. Origins of asymmetry in the CNS. Semin. Cell Dev. Biol. 20, 485–490 (2009).
Chiandetti, C. & Vallortigara, G. Distinct effect of early and late embryonic light-stimulation on chicks’ lateralization. Neuroscience 414, 1–7 (2019).
Chiandetti, C. Pseudoneglect and embryonic light stimulation in the avian brain. Behav. Neurosci. 125, 775–782 (2011).
Güntürkün, O., Hellmann, B., Melsbach, G. & Prior, H. Asymmetries of representation in the visual system of pigeons. Neuroreport 9, 4127–4130 (1998).
Morandi-Raikova, A. & Mayer, U. Selective activation of the right hippocampus during navigation by spatial cues in domestic chicks (Gallus gallus). Neurobiol. Learn. Mem. 177, 107344 (2021).
Morandi-Raikova, A. & Mayer, U. The effect of monocular occlusion on hippocampal c-Fos expression in domestic chicks (Gallus gallus). Sci. Rep. 10, 7205 (2020).
Manns, M. & Ströckens, F. Functional and structural comparison of visual lateralization in birds — similar but still different. Front. Psychol. 5, 206 (2014).
Rogers, L. J. Light experience and asymmetry of brain function in chickens. Nature 297, 223–225 (1982).
Rogers, L. J. & Sink, H. S. Transient asymmetry in the projections of the rostral thalamus to the visual hyperstriatum of the chicken, and reversal of its direction by light exposure. Exp. Brain Res. 70, 378–384 (1988).
Rogers, L. J. & Deng, C. Light experience and lateralization of the two visual pathways in the chick. Behav. Brain Res. 98, 277–287 (1999).
Costalunga, G., Kobylkov, D., Rosa Salva, O., Vallortigara, G. & Mayer, U. Light-incubation effects on lateralisation of single unit responses in the visual Wulst of domestic chicks. Brain Struct. Funct. 227, 497–513 (2022).
Costalunga, G. et al. Responses in the left and right entopallium are differently affected by light stimulation in embryo. iScience 27, 109268 (2024).
Vallortigara, G., Cozzutti, C., Tommasi, L. & Rogers, L. J. How birds use their eyes: opposite left-right specialization for the lateral and frontal visual hemifield in the domestic chick. Curr. Biol. 11, 29–33 (2001).
Vallortigara, G. & Rogers, L. J. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575–633 (2005).
Rugani, R., Macchinizzi, M., Zhang, Y. & Regolin, L. Hatching with numbers: pre-natal light exposure affects number sense and the mental number line in young domestic chicks. eLife 14, RP106356 (2025).
Loconsole, M. & Regolin, L. Are prime numbers special? Insights from the life sciences. Biol. Direct 17, 11 (2022).
Sinha, S. The Fibonacci numbers and its amazing applications. Int. J. Eng. Sci. Invent. 6, 7–14 (2019).
Lehmann-Ziebarth, N. et al. Evolution of periodicity in periodical cicadas. Ecology 86, 3200–3211 (2005).
Sacks, O. W. The Man Who Mistook His Wife for a Hat (Picador, 1986).
Anderson, M., O’Connor, N. & Hermelin, B. A specific calculating ability. Intelligence 26, 383–403 (1998).
Hermelin, B. & O’Connor, N. Factors and primes: a specific numerical ability. Psychol. Med. 20, 163–169 (1990).
Welling, H. Prime number identification in idiots savants: can they calculate them? J. Autism Dev. Disord. 24, 199–207 (1994).
Vallortigara, G. in The Oxford Handbook of Comparative Cognition (eds Wasserman, E. A. & Zentall, T. R.) 48–66 (Oxford Univ. Press, 2012).
Clara, E., Regolin, L. & Vallortigara, G. Preference for symmetry is experience dependent in newborn chicks (Gallus gallus). J. Exp. Psychol. Anim. Behav. Process. 33, 12–20 (2007).
Forsman, A. & Herrström, J. Asymmetry in size, shape, and color impairs the protective value of conspicuous color patterns. Behav. Ecol. 15, 141–147 (2004).
Loconsole, M., De Agrò, M. & Regolin, L. Young chicks rely on symmetry/asymmetry in perceptual grouping to discriminate sets of elements. Proc. R. Soc. B 288, 20211570 (2021).
Jackson, P. S. & Bateson, P. P. Imprinting and exploration of slight novelty in chicks. Nature 251, 609–610 (1974).
Rugani, R., Cavazzana, A., Vallortigara, G. & Regolin, L. One, two, three, four, or is there something more? Numerical discrimination in day-old domestic chicks. Anim. Cogn. 16, 557–564 (2013).
Rugani, R., Vallortigara, G. & Regolin, L. From small to large: numerical discrimination by young domestic chicks (Gallus gallus). J. Comp. Psychol. 128, 163–171 (2014).
Wertheimer, M. in A Source Book of Gestalt Psychology (ed. Ellis, W. D.) 71–88 (Kegan Paul, Trench, Trubner & Company, 1938).
Geraci, A., Loconsole, M. & Regolin, L. A symmetry-based mechanism for perceptual grouping in preverbal infants. Sci. Rep. 15, 5035 (2025).
Szabó, E. et al. Young domestic chicks spontaneously represent the absence of objects. eLife 11, e67208 (2022).
Pepperberg, I. M. in Mathematical Cognition and Learning (eds Geary, D. C. et al.) 67–89 (Elsevier, 2015).
Pepperberg, I. M. Numerical competence in an African gray parrot (Psittacus erithacus). J. Comp. Psychol. 108, 36–44 (1994).
Merritt, D. J., Rugani, R. & Brannon, E. M. Empty sets as part of the numerical continuum: conceptual precursors to the zero concept in rhesus monkeys. J. Exp. Psychol. Gen. 138, 258–269 (2009).
Nieder, A. Representing something out of nothing: the dawning of zero. Trends Cogn. Sci. 20, 830–842 (2016).
Pepperberg, I. M. Proficient performance of a conjunctive, recursive task by an African gray parrot (Psittacus erithacus). J. Comp. Psychol. 106, 295–305 (1992).
Pepperberg, I. M. A review of the model/rival (M/R) technique for training interspecies communication and its use in behavioral research. Animals 11, 2479 (2021).
Pepperberg, I. M. Animal language studies: what happened? Psychon. Bull. Rev. 24, 181–185 (2017).
Pepperberg, I. M. & Sherman, D. V. Use of two-trainer interactive modeling as a potential means to engender social behavior in children with various disabilities. Int. J. Comp. Psychol. 15, 138–153 (2002).
Pepperberg, I. M. & Sherman, D. Proposed use of two-part interactive modeling as a means to increase functional skills in children with a variety of disabilities. Teach. Learn. Med. 12, 213–220 (2000).
Wynne, C. Psychology’s pet subject. Nature 455, 864–865 (2008).
Pepperberg, I. M. The Alex Studies: Cognitive and Communicative Abilities of Grey Parrots (Harvard Univ. Press, 2009).
Acknowledgements
The authors acknowledge support from PRIN (Progetti di Rilevante Interesse Nazionale - Projects of Relevant National Interest) 2022 PNRR (Piano Nazionale di Ripresa e Resilienza - National Plan for Recovery and Resilience) (grant P2022TKY7B to L.R. and R.R.) and PRIN 2022 (grant 202254RHRT to R.R.). This project was supported by funding from the European Research Council under the European Union’s Horizon 2020 Research and Innovation programme (grant 833504 SPANUMBRA). M.L. was funded by the European Union (NextGenerationEU) and by the University of Padua under the 2023 STARS Grants@Unipd programme (project CROSS).
Author information
Authors and Affiliations
Contributions
L.R., M.L., O.R.-S. and R.R. conceptualized the review. L.R., M.L., O.R.-S., K.B., M.M., A.F. and R.R. performed the literature search. O.R.-S., K.B., M.M. and R.R. created drafts of the figures. L.R., M.L., O.R.-S. and R.R. wrote the initial draft of the manuscript. All authors critically reviewed the manuscript and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Psychology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Regolin, L., Loconsole, M., Rosa-Salva, O. et al. Numerical cognition in birds. Nat Rev Psychol 4, 576–590 (2025). https://doi.org/10.1038/s44159-025-00480-8
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44159-025-00480-8