Abstract
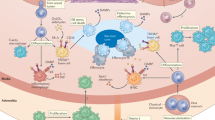
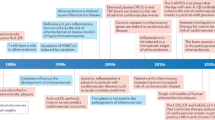
Atherosclerotic cardiovascular disease is a major cause of disability and death worldwide. Most therapeutic approaches target traditional risk factors but ignore the fundamental role of the immune system. This is a huge unmet need. Recent evidence indicates that reducing inflammation may limit cardiovascular events. However, the concomitant increase in the risk of life-threatening infections is a major drawback. In this context, targeting adaptive immunity could constitute a highly effective and safer approach. In this Review, we address the why and how of the immuno-cardiovascular unit, in health and in atherosclerotic disease. We review and discuss fundamental mechanisms that ensure immune tolerance to cardiovascular tissue, and examine how their disruption promotes disease progression. We identify promising strategies to manipulate the adaptive immune system for patient benefit, including novel biologics and RNA-based vaccination strategies. Finally, we advocate for establishing a molecular classification of atherosclerosis as an important milestone in our quest to radically change the understanding and treatment of atherosclerotic disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Boren, J. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 41, 2313–2330 (2020).
Roy, P., Orecchioni, M. & Ley, K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-021-00584-1 (2021).
Rieckmann, M. et al. Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses. J. Clin. Invest. 129, 4922–4936 (2019).
Kyaw, T. et al. Alarmin-activated B cells accelerate murine atherosclerosis after myocardial infarction via plasma cell-immunoglobulin-dependent mechanisms. Eur. Heart J. 42, 938–947 (2021). Mouse study demonstrating the role of GC B cells and antibodies in accelerated atherosclerosis post-MI, with potential implications for secondary prevention.
Zhao, T. X. & Mallat, Z. Targeting the immune system in atherosclerosis: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73, 1691–1706 (2019).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Hansson, G. K., Holm, J. & Jonasson, L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am. J. Pathol. 135, 169–175 (1989).
Palinski, W. et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl Acad. Sci. USA 86, 1372–1376 (1989).
Stemme, S. et al. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl Acad. Sci. USA 92, 3893–3897 (1995).
Sage, A. P., Tsiantoulas, D., Binder, C. J. & Mallat, Z. The role of B cells in atherosclerosis. Nat. Rev. Cardiol. 16, 180–196 (2019).
Zernecke, A. et al. Meta-analysis of leukocyte diversity in atherosclerotic mouse aortas. Circ. Res. 127, 402–426 (2020).
Wade, N. S. & Major, A. S. The problem of accelerated atherosclerosis in systemic lupus erythematosus: insights into a complex co-morbidity. Thromb. Haemost. 106, 849–857 (2011).
Huan, T. et al. A systems biology framework identifies molecular underpinnings of coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 33, 1427–1434 (2013).
Mauersberger, C., Schunkert, H. & Sager, H. B. Inflammation-related risk loci in genome-wide association studies of coronary artery disease. Cells 10, 440 (2021).
Bjorkbacka, H. et al. Weak associations between human leucocyte antigen genotype and acute myocardial infarction. J. Intern. Med. 268, 50–58 (2010).
Bjorkegren, J. L. M., Kovacic, J. C., Dudley, J. T. & Schadt, E. E. Genome-wide significant loci: how important are they? Systems genetics to understand heritability of coronary artery disease and other common complex disorders. J. Am. Coll. Cardiol. 65, 830–845 (2015).
Dansky, H. M., Charlton, S. A., Harper, M. M. & Smith, J. D. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc. Natl Acad. Sci. USA 94, 4642–4646 (1997).
Drobni, Z. D. et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 142, 2299–2311 (2020).
Mohanta, S. K. et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature (in the press). Detailed description of neuro–immune–vascular interactions in the adventitia and outer media of large arteries, establishing an artery–brain crosstalk and the impact thereof on maintenance of artery tertiary lymphoid organs and the progression of atherosclerosis.
Wick, G., Jakic, B., Buszko, M., Wick, M. C. & Grundtman, C. The role of heat shock proteins in atherosclerosis. Nat. Rev. Cardiol. 11, 516–529 (2014).
Cros, J. et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33, 375–386 (2010).
Narasimhan, P. B., Marcovecchio, P., Hamers, A. A. J. & Hedrick, C. C. Nonclassical monocytes in health and disease. Annu. Rev. Immunol. 37, 439–456 (2019).
Williams, J. W. et al. Limited proliferation capacity of aortic intima resident macrophages requires monocyte recruitment for atherosclerotic plaque progression. Nat. Immunol. 21, 1194–1204 (2020).
Lim, H. Y. et al. Hyaluronan receptor LYVE-1-expressing macrophages maintain arterial tone through hyaluronan-mediated regulation of smooth muscle cell collagen. Immunity 49, 1191 (2018).
Zhou, J. et al. CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses. J. Exp. Med. 207, 1951–1966 (2010).
Hernandez, G. E. et al. Aortic intimal resident macrophages are essential for maintenance of the non-thrombogenic intravascular state. Nat. Cardiovasc. Res. 1, 67–84 (2022).
Ma-Krupa, W. et al. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J. Exp. Med. 199, 173–183 (2004).
Galkina, E. et al. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J. Exp. Med. 203, 1273–1282 (2006).
Jackson-Jones, L. H. et al. Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat. Commun. 7, 12651 (2016).
Srikakulapu, P. et al. Perivascular adipose tissue harbors atheroprotective IgM-producing B cells. Front. Physiol. 8, 719 (2017).
Newland, S. A. et al. Type-2 innate lymphoid cells control the development of atherosclerosis in mice. Nat. Commun. 8, 15781 (2017).
Binder, C. J. et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 114, 427–437 (2004).
Cardilo-Reis, L. et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 4, 1072–1086 (2012).
Tellides, G. & Pober, J. S. Inflammatory and immune responses in the arterial media. Circ. Res. 116, 312–322 (2015).
Roozendaal, R. & Mebius, R. E. Stromal cell–immune cell interactions. Annu Rev Immunol 29, 23–43 (2011).
Chan, T. D. et al. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity 37, 893–904 (2012).
Krautler, N. J. et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell 150, 194–206 (2012).
Lin, Z. et al. Deep sequencing of the T cell receptor beta repertoire reveals signature patterns and clonal drift in atherosclerotic plaques and patients. Oncotarget 8, 99312–99322 (2017).
Hamze, M. et al. Characterization of resident B cells of vascular walls in human atherosclerotic patients. J. Immunol. 191, 3006–3016 (2013).
Nilsson, J. & Hansson, G. K. Vaccination strategies and immune modulation of atherosclerosis. Circ. Res. 126, 1281–1296 (2020).
MacRitchie, N. et al. The aorta can act as a site of naive CD4+ T-cell priming. Cardiovasc. Res. 116, 306–316 (2020).
Li, J. et al. CCR5+T-bet+FoxP3+ effector CD4 T cells drive atherosclerosis. Circ. Res. 118, 1540–1552 (2016).
Vila-Caballer, M. et al. Disruption of the CCL1–CCR8 axis inhibits vascular Treg recruitment and function and promotes atherosclerosis in mice. J. Mol. Cell. Cardiol. 132, 154–163 (2019).
Tsilingiri, K. et al. Oxidized low-density lipoprotein receptor in lymphocytes prevents atherosclerosis and predicts subclinical disease. Circulation 139, 243–255 (2019).
Kimura, T. et al. Regulatory CD4+ T cells recognize major histocompatibility complex class II molecule-restricted peptide epitopes of apolipoprotein B. Circulation 138, 1130–1143 (2018). Identification of an ApoB peptide as the first Treg cell epitope in human and mouse atherosclerosis.
Maganto-Garcia, E., Tarrio, M. L., Grabie, N., Bu, D. X. & Lichtman, A. H. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation 124, 185–195 (2011).
Wolf, D. et al. Pathogenic autoimmunity in atherosclerosis evolves from initially protective apolipoprotein B100-reactive CD+ T-regulatory cells. Circulation 142, 1279–1293 (2020).
Mailer, R. K. W., Gistera, A., Polyzos, K. A., Ketelhuth, D. F. J. & Hansson, G. K. Hypercholesterolemia induces differentiation of regulatory T cells in the liver. Circ. Res. 120, 1740–1753 (2017).
Almanzar, G. et al. Autoreactive HSP60 epitope-specific T-cells in early human atherosclerotic lesions. J Autoimmun 39, 441–450 (2012).
Sage, A. P. et al. X-Box binding protein-1 dependent plasma cell responses limit the development of atherosclerosis. Circ. Res. 121, 270–281 (2017).
Sage, A. P. et al. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic T cell immunity. Circulation 130, 1363–1373 (2014).
Clement, M. et al. Deletion of IRF8 (interferon regulatory factor 8)-dependent dendritic cells abrogates proatherogenic adaptive immunity. Circ. Res. 122, 813–820 (2018).
Zernecke, A. Dendritic cells in atherosclerosis: evidence in mice and humans. Arterioscler. Thromb. Vasc. Biol. 35, 763–770 (2015).
Bonacina, F. et al. Myeloid apolipoprotein E controls dendritic cell antigen presentation and T cell activation. Nat. Commun. 9, 3083 (2018).
Clement, M. et al. Impaired autophagy in CD11b+ dendritic cells expands CD4+ regulatory T cells and limits atherosclerosis in mice. Circ. Res. 125, 1019–1034 (2019).
Lacy, M. et al. Cell-specific and divergent roles of the CD40L–CD40 axis in atherosclerotic vascular disease. Nat. Commun. 12, 3754 (2021).
Baardman, J. & Lutgens, E. Regulatory T cell metabolism in atherosclerosis. Metabolites 10, 279 (2020).
Gaddis, D. E. et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat. Commun. 9, 1095 (2018).
Bailey-Bucktrout, S. L. et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39, 949–962 (2013).
Butcher, M. J. et al. Atherosclerosis-driven Treg plasticity results in formation of a dysfunctional subset of plastic IFNγ+ TH1/Tregs. Circ. Res. 119, 1190–1203 (2016).
Taleb, S., Tedgui, A. & Mallat, Z. IL-17 and TH17 cells in atherosclerosis: subtle and contextual roles. Arterioscler Thromb. Vasc. Biol. 35, 258–264 (2015).
Fernandez, D. M. et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 25, 1576–1588 (2019). Single-cell analyses of human carotid plaques, demonstrating the complexity and heterogeneity of infiltrating adaptive immune cells and their activation in symptomatic disease.
Depuydt, M. A. C. et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ. Res. 127, 1437–1455 (2020).
Getz, G. S. & Reardon, C. A. Natural killer T cells in atherosclerosis. Nat. Rev. Cardiol. 14, 304–314 (2017).
He, S. et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature 566, 115–119 (2019).
Schafer, S. & Zernecke, A. CD8+ T cells in atherosclerosis. Cells 10, 37 (2020).
Dimayuga, P. C. et al. Identification of apoB-100 peptide-specific CD8+ T cells in atherosclerosis. J. Am. Heart Assoc. 6, e005318 (2017).
Clement, M. et al. Control of the T follicular helper-germinal center B-cell axis by CD8+ regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation 131, 560–570 (2015).
Hu, D. et al. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin beta receptors. Immunity 42, 1100–1115 (2015).
Srikakulapu, P. et al. Artery tertiary lymphoid organs control multilayered territorialized atherosclerosis B-cell responses in aged ApoE–/– mice. Arterioscler. Thromb. Vasc. Biol. 36, 1174–1185 (2016).
Tay, C. et al. Follicular B cells promote atherosclerosis via T cell-mediated differentiation into plasma cells and secreting pathogenic immunoglobulin G. Arterioscler. Thromb. Vasc. Biol. 38, e71–e84 (2018).
Chou, M. Y. et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Invest. 119, 1335–1349 (2009).
Nus, M. et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat. Med. 23, 601–610 (2017).
Ait-Oufella, H. et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 207, 1579–1587 (2010).
Kyaw, T. et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J. Immunol. 185, 4410–4419 (2010).
Sage, A. P. et al. BAFF receptor deficiency reduces the development of atherosclerosis in mice—brief report. Arterioscler. Thromb. Vasc. Biol. 32, 1573–1576 (2012).
Centa, M. et al. Acute loss of apolipoprotein E triggers an autoimmune response that accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 38, e145–e158 (2018).
Hutchinson, M. A. et al. Auto-antibody production during experimental atherosclerosis in ApoE–/– mice. Front. Immunol. 12, 695220 (2021).
Centa, M. et al. Germinal center-derived antibodies promote atherosclerosis plaque size and stability. Circulation 139, 2466–2482 (2019). Demonstration of the effect of germinal-center-derived antibodies in promoting atherosclerotic lesion size and modulating plaque stability in mice.
Crane, E. D. et al. Anti-GRP78 autoantibodies induce endothelial cell activation and accelerate the development of atherosclerotic lesions. JCI Insight 3, e99363 (2018).
Porsch, F., Mallat, Z. & Binder, C. J. Humoral immunity in atherosclerosis and myocardial infarction: from B cells to antibodies. Cardiovasc. Res. 117, 2544–2562 (2021).
Lorenzo, C. et al. ALDH4A1 is an atherosclerosis auto-antigen targeted by protective antibodies. Nature 589, 287–292 (2021). An elegant high-throughput approach for the identification and evaluation of novel B cell antigens in atherosclerosis using single-cell analyses, mass spectrometry and recombinant technology.
Gistera, A. et al. Low-density lipoprotein-reactive T cells regulate plasma cholesterol levels and development of atherosclerosis in humanized hypercholesterolemic mice. Circulation 138, 2513–2526 (2018).
Rhoads, J. P. et al. Oxidized low-density lipoprotein immune complex priming of the Nlrp3 inflammasome involves TLR and FcγR cooperation and is dependent on CARD9. J. Immunol. 198, 2105–2114 (2017).
van den Berg, V. J. et al. Anti-Oxidized LDL antibodies and coronary artery disease: a systematic review. Antioxidants 8, 84 (2019).
Papac-Milicevic, N., Busch, C. J. & Binder, C. J. Malondialdehyde epitopes as targets of immunity and the implications for atherosclerosis. Adv. Immunol. 131, 1–59 (2016).
Schiopu, A. et al. Recombinant antibodies to an oxidized low-density lipoprotein epitope induce rapid regression of atherosclerosis in apobec-1–/–/low-density lipoprotein receptor–/– mice. J. Am. Coll. Cardiol. 50, 2313–2318 (2007).
de Vries, M. R. et al. Identification of IgG1 isotype phosphorylcholine antibodies for the treatment of inflammatory cardiovascular diseases. J. Intern. Med. 290, 141–156 (2021).
Que, X. et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 558, 301–306 (2018). Overexpression of a single-chain variable fragment of E06, which binds to the phosphocholine headgroup, reduces systemic inflammation, atherosclerosis progression, aortic stenosis and hepatic steatosis in mice.
Bagchi-Chakraborty, J. et al. B cell Fcγ receptor IIb modulates atherosclerosis in male and female mice by controlling adaptive germinal center and innate B-1-cell responses. Arterioscler. Thromb. Vasc. Biol. 39, 1379–1389 (2019).
Tsiantoulas, D. et al. Increased plasma IgE accelerate atherosclerosis in secreted IgM deficiency. Circ. Res. 120, 78–84 (2017).
Zhang, X. et al. IgE contributes to atherosclerosis and obesity by affecting macrophage polarization, macrophage protein network, and foam cell formation. Arterioscler. Thromb. Vasc. Biol. 40, 597–610 (2020).
Tay, C. et al. B-cell-specific depletion of tumour necrosis factor alpha inhibits atherosclerosis development and plaque vulnerability to rupture by reducing cell death and inflammation. Cardiovasc. Res. 111, 385–397 (2016).
Hilgendorf, I. et al. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation 129, 1677–1687 (2014).
Lavine, K. J. et al. The macrophage in cardiac homeostasis and disease: JACC macrophage in CVD series (part 4). J. Am. Coll. Cardiol. 72, 2213–2230 (2018).
Van der Borght, K. et al. Myocardial infarction primes autoreactive T cells through activation of dendritic cells. Cell Rep. 18, 3005–3017 (2017). This study shows that cardiac cDC1s drive the proliferation and differentiation of cardiac α-myosin-specific Treg cells at steady state, while cDC2s drive the proliferation of autoreactive T cells and their differentiation into effector cells after myocardial infarction.
Lv, H. et al. Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. J. Clin. Invest. 121, 1561–1573 (2011).
Tang, T. T. et al. Pathologic T-cell response in ischaemic failing hearts elucidated by T-cell receptor sequencing and phenotypic characterization. Eur. Heart J. 40, 3924–3933 (2019). Bulk TCR sequencing on heart-infiltrating T cells reveals TCR clonotypes shared between ischemic failing hearts of several patients, with a dominance of CD4+ TH1 cells and cytotoxic CD8+ T cells.
Xia, N. et al. A unique population of regulatory T cells in heart potentiates cardiac protection from myocardial infarction. Circulation 142, 1956–1973 (2020).
Komarowska, I. et al. Hepatocyte growth factor receptor c-Met instructs T cell cardiotropism and promotes T cell migration to the heart via autocrine chemokine release. Immunity 42, 1087–1099 (2015).
Dobaczewski, M., Xia, Y., Bujak, M., Gonzalez-Quesada, C. & Frangogiannis, N. G. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am. J. Pathol. 176, 2177–2187 (2010).
DeBerge, M. et al. Monocytes prime autoreactive T cells after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 318, H116–H123 (2020).
Lee, J. S. et al. Conventional dendritic cells impair recovery after myocardial infarction. J. Immunol. 201, 1784–1798 (2018).
Boag, S. E. et al. T lymphocytes and fractalkine contribute to myocardial ischemia/reperfusion injury in patients. J. Clin. Invest. 125, 3063–3076 (2015).
Yang, Z. et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation 114, 2056–2064 (2006).
Santos-Zas, I. et al. Cytotoxic CD8+ T cells promote granzyme B-dependent adverse post-ischemic cardiac remodeling. Nat. Commun. 12, 1483 (2021). Evidence for a deleterious role of CD8+ T cells following acute MI in mice and pigs through the production of granzyme B, with circulating levels of the latter being predictive of 1-year mortality in people with acute MI.
Forte, E. et al. Cross-priming dendritic cells exacerbate immunopathology after ischemic tissue damage in the heart. Circulation 143, 821–836 (2021).
Hoffmann, J. et al. Myocardial ischemia and reperfusion leads to transient CD8 immune deficiency and accelerated immunosenescence in CMV-seropositive patients. Circ. Res. 116, 87–98 (2015).
Chen, X. M. et al. Gene expression pattern of TCR repertoire and alteration expression of IL-17A gene of γδ T cells in patients with acute myocardial infarction. J. Transl. Med. 16, 189 (2018).
Klingenberg, R. et al. Clonal restriction and predominance of regulatory T cells in coronary thrombi of patients with acute coronary syndromes. Eur. Heart J. 36, 1041–1048 (2015).
Xia, N. et al. Activated regulatory T-cells attenuate myocardial ischaemia/reperfusion injury through a CD39-dependent mechanism. Clin. Sci. 128, 679–693 (2015).
Wang, Y. et al. C-X-C motif chemokine receptor 4 blockade promotes tissue repair after myocardial infarction by enhancing regulatory T cell mobilization and immune-regulatory function. Circulation 139, 1798–1812 (2019).
Hofmann, U. et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125, 1652–1663 (2012).
Matsumoto, K. et al. Regulatory T lymphocytes attenuate myocardial infarction-induced ventricular remodeling in mice. Int. Heart J. 52, 382–387 (2011).
Weirather, J. et al. Foxp3+CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ. Res. 115, 55–67 (2014).
Wigren, M. et al. Low levels of circulating CD4+FoxP3+ T cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arterioscler. Thromb. Vasc. Biol. 32, 2000–2004 (2012).
Zacchigna, S. et al. Paracrine effect of regulatory T cells promotes cardiomyocyte proliferation during pregnancy and after myocardial infarction. Nat. Commun. 9, 2432 (2018). Evidence for a role of Treg cells in promoting fetal and maternal cardiomyocyte proliferation after MI in mice, with a significant impact on infarct size and cardiac contractility.
Bansal, S. S. et al. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 139, 206–221 (2019).
Adamo, L. et al. Myocardial B cells are a subset of circulating lymphocytes with delayed transit through the heart. JCI Insight 5, e134700 (2020). Detailed characterization of B cells in naive mouse hearts and their circulating origin and transit properties through the heart.
Rocha-Resende, C. et al. Developmental changes in myocardial B cells mirror changes in B cells associated with different organs. JCI Insight 5, e139377 (2020).
Horckmans, M. et al. Pericardial adipose tissue regulates granulopoiesis, fibrosis, and cardiac function after myocardial infarction. Circulation 137, 948–960 (2018). Identification of lymphoid cell clusters in human and murine epicardial adipose tissue and their role in regulating cardiac remodeling post-MI.
Wu, L. et al. IL-10-producing B cells are enriched in murine pericardial adipose tissues and ameliorate the outcome of acute myocardial infarction. Proc. Natl Acad. Sci. USA 116, 21673–21684 (2019).
Rocha-Resende, C., Pani, F. & Adamo, L. B cells modulate the expression of MHC-II on cardiac CCR2– macrophages. J. Mol. Cell. Cardiol. 157, 98–103 (2021).
Zouggari, Y. et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 19, 1273–1280 (2013).
Sun, Y. et al. Splenic marginal zone B lymphocytes regulate cardiac remodeling after acute myocardial infarction in mice. J. Am. Coll. Cardiol. (in the press). Identification of MZ B cells as mediators of adverse cardiac remodeling post-MI and the contribution of miR21-dependent upregulation of HIF1α to this effect.
Haas, M. S. et al. Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc Res 87, 618–627 (2010).
Kaya, Z., Leib, C. & Katus, H. A. Autoantibodies in heart failure and cardiac dysfunction. Circ. Res. 110, 145–158 (2012).
Zhao, T. X. et al. Rituximab in patients with acute ST-elevation myocardial infarction (RITA-MI): an experimental medicine safety study. Cardiovasc. Res. 118, 872–882 (2021). Treatment with rituximab is safe when given in the acute ST-elevation MI setting and substantially alters circulating B cell subsets.
Tsiantoulas, D. et al. B cell-activating factor neutralization aggravates atherosclerosis. Circulation 138, 2263–2273 (2018).
Lehrer-Graiwer, J. et al. FDG-PET imaging for oxidized LDL in stable atherosclerotic disease: a phase II study of safety, tolerability, and anti-inflammatory activity. JACC Cardiovasc. Imaging 8, 493–494 (2015).
Binder, C. J. et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 9, 736–743 (2003).
Binder, C. J., Papac-Milicevic, N. & Witztum, J. L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 16, 485–497 (2016).
Houben, T. et al. Pneumococcal immunization reduces neurological and hepatic symptoms in a mouse model for Niemann–Pick type C1 disease. Front. Immunol. 9, 3089 (2018).
Grievink, H. W. et al. The effect of a 13-valent conjugate pneumococcal vaccine on circulating antibodies against oxidized LDL and phosphorylcholine in man, a randomized placebo-controlled clinical trial. Biology 9, 345 (2020).
Ren, S. et al. Rationale and design of a randomized controlled trial of pneumococcal polysaccharide vaccine for prevention of cardiovascular events: The Australian Study for the Prevention through Immunization of Cardiovascular Events (AUSPICE). Am. Heart J. 177, 58–65 (2016).
Ren, S. et al. Effect of the adult pneumococcal polysaccharide vaccine on cardiovascular disease: a systematic review and meta-analysis. Open Heart 2, e000247 (2015).
Zhao, T. X., Newland, S. A. & Mallat, Z. 2019 ATVB Plenary Lecture: interleukin-2 therapy in cardiovascular disease: the potential to regulate innate and adaptive immunity. Arterioscler. Thromb. Vasc. Biol. 40, 853–864 (2020).
Zhao, T. X. et al. Regulatory T cell response to low-dose interleukin-2 in ischemic heart disease. NEJM Evidence 1, EVIDoa2100009 (2021). In this phase 1b/2a study, low-dose IL-2 expanded Treg cells without adverse events of major concern, and single-cell RNA sequencing demonstrated the engagement of distinct pathways and cell–cell interactions after low-dose IL-2.
Yu, X. et al. Innate lymphoid cells promote recovery of ventricular function after myocardial infarction. J. Am. Coll. Cardiol. 78, 1127–1142 (2021).
Trotta, E. et al. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat. Med. 24, 1005–1014 (2018).
Khoryati, L. et al. An IL-2 mutein engineered to promote expansion of regulatory T cells arrests ongoing autoimmunity in mice. Sci. Immunol. 5, eaba5264 (2020).
Sockolosky, J. T. et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine–receptor complexes. Science 359, 1037–1042 (2018).
Ait-Oufella, H. et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 12, 178–180 (2006).
Herbin, O. et al. Regulatory T-cell response to apolipoprotein B100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 32, 605–612 (2012).
Kimura, T. et al. Atheroprotective vaccination with MHC-II-restricted ApoB peptides induces peritoneal IL-10-producing CD4 T cells. Am. J. Physiol. Heart Circ. Physiol. 312, H781–H790 (2017).
Tiret, L. et al. Genetic analysis of the interleukin-18 system highlights the role of the interleukin-18 gene in cardiovascular disease. Circulation 112, 643–650 (2005).
Sheedy, F. J. et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Pattarabanjird, T., Li, C. & McNamara, C. B cells in atherosclerosis: mechanisms and potential clinical applications. JACC Basic Transl. Sci. 6, 546–563 (2021).
Kyaw, T. et al. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ. Res. 109, 830–840 (2011).
Rosenfeld, S. M. et al. B-1b cells secrete atheroprotective IgM and attenuate atherosclerosis. Circ. Res. 117, e28–e39 (2015).
Srikakulapu, P. et al. Chemokine receptor-6 promotes B-1 cell trafficking to perivascular adipose tissue, local IgM production and atheroprotection. Front. Immunol. 12, 636013 (2021).
Tsiantoulas, D. et al. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J. Lipid Res. 56, 440–448 (2015).
Obermayer, G. et al. Natural IgM antibodies inhibit microvesicle-driven coagulation and thrombosis. Blood 137, 1406–1415 (2021).
Perry, H. M. et al. Helix-loop-helix factor inhibitor of differentiation 3 regulates interleukin-5 expression and B-1a B cell proliferation. Arterioscler. Thromb. Vasc. Biol. 33, 2771–2779 (2013).
Doring, Y. et al. B-cell-specific CXCR4 protects against atherosclerosis development and increases plasma IgM levels. Circ. Res. 126, 787–788 (2020).
Gruber, S. et al. Sialic acid-binding immunoglobulin-like lectin G promotes atherosclerosis and liver inflammation by suppressing the protective functions of B-1 cells. Cell Rep. 14, 2348–2361 (2016).
Grasset, E. K. et al. Sterile inflammation in the spleen during atherosclerosis provides oxidation-specific epitopes that induce a protective B-cell response. Proc. Natl Acad. Sci. USA 112, E2030–E2038 (2015).
Ensan, S. et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1+ precursors and circulating monocytes immediately after birth. Nat. Immunol. 17, 159–168 (2016).
Tsiantoulas, D. et al. APRIL limits atherosclerosis by binding to heparan sulfate proteoglycans. Nature 597, 92–96 (2021). Identification of a non-canonical function for the B cell cytokine APRIL with critical implications for vascular homeostasis and atherosclerotic cardiovascular disease.
Ryu, H. et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat. Immunol. 19, 583–593 (2018).
Zhivaki, D. & Kagan, J. C. Innate immune detection of lipid oxidation as a threat assessment strategy. Nat. Rev. Immunol. 5, eaba5264 (2021).
York, A. G. et al. Limiting cholesterol biosynthetic flux spontaneously engages type I IFN signaling. Cell 163, 1716–1729 (2015).
Ridker, P. M. How common is residual inflammatory risk? Circ. Res. 120, 617–619 (2017).
Acknowledgements
Z.M. is supported by the British Heart Foundation (RCAM/104, RCAM-659, RRCAM.163), the British Heart Foundation Center for Research Excellence (RE/18/1/34212), and the NIHR Cambridge Biomedical Research Centre (RG85315). C.J.B. is supported by grants from the Austrian Science Fund (FWF SFB F54) and the Vienna Science and Technology Fund (LS18-090). Z.M. and C.J.B. are supported by the Leducq Foundation (Transatlantic Network of Excellence; TNE-20CVD03).
Author information
Authors and Affiliations
Contributions
Z. M. and C. J. B. wrote the manuscript and reviewed it for important intellectual content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Jan Nilsson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mallat, Z., Binder, C.J. The why and how of adaptive immune responses in ischemic cardiovascular disease. Nat Cardiovasc Res 1, 431–444 (2022). https://doi.org/10.1038/s44161-022-00049-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44161-022-00049-1
This article is cited by
-
Suppression of CSF2RA macrophage polarisation impacts pathological cardiac remodelling in mice
Scientific Reports (2026)
-
T cells in cardiovascular disease: solved and unsolved mysteries
Science China Life Sciences (2026)
-
Translating Nobel Prize-winning Treg cell science into cardiovascular therapy
Nature Cardiovascular Research (2025)
-
Apolipoprotein B-containing lipoproteins in atherogenesis
Nature Reviews Cardiology (2025)
-
Genetic Insights into the Connection Between Antibody Responses to Infectious Diseases Agents and Atrial Fibrillation: A Bidirectional Two-Sample Mendelian Randomization Study
Indian Journal of Microbiology (2025)