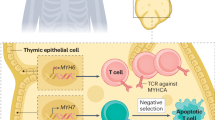
Abstract
In this Review, we present a comprehensive analysis of preclinical models used to study immune checkpoint inhibitor-associated myocarditis (hereafter ICI-myocarditis), a potentially lethal immune-related adverse event. We begin by providing an overview of immune checkpoint inhibitors, highlighting how their efficacy in cancer treatment is counterbalanced by their predisposition to cause immune-related adverse events. Next, we draw from human data to identify disease features that an effective mouse model should ideally mimic. After that, we present a critical evaluation of a wide variety of existing mouse models including genetic, pharmacological and humanized models. We summarize insights gathered about the underlying mechanisms of ICI-myocarditis and the role of mouse models in these discoveries. We conclude with a perspective on the future of preclinical models, highlighting potential model improvements and research directions that could strengthen our understanding of ICI-myocarditis, ultimately improving patient outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Haslam, A. & Prasad, V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw. Open 2, e192535 (2019).
Tan, S., Day, D., Nicholls, S. J. & Segelov, E. Immune checkpoint inhibitor therapy in oncology. JACC CardioOncol. 4, 579–597 (2022).
Pauken, K. E., Dougan, M., Rose, N. R., Lichtman, A. H. & Sharpe, A. H. Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol. 40, 511–523 (2019).
Ramos-Casals, M. et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 6, 38 (2020).
Postow Michael, A., Sidlow, R. & Hellmann Matthew, D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Wang, D. Y. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4, 1721–1728 (2018). Meta-analysis describing incidence and mortality rates of various irAEs, including ICI-myocarditis.
Johnson Douglas, B. et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 375, 1749–1755 (2016). Fundamental work describing two patients with fulminant ICI-myocarditis, with molecular characterization of the pathophysiology.
Mahmood, S. S. et al. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 71, 1755–1764 (2018).
Al-Kindi, S. G. & Oliveira, G. H. Reporting of immune checkpoint inhibitor-associated myocarditis. Lancet 392, 382–383 (2018).
Moslehi, J. J., Salem, J.-E., Sosman, J. A., Lebrun-Vignes, B. & Johnson, D. B. Rapid increase in reporting of fatal immune checkpoint inhibitor associated myocarditis. Lancet 391, 933 (2018).
Salem, J.-E. et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 19, 1579–1589 (2018).
Mestas, J. & Hughes, C. C. W. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Hu, J.-R. et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 115, 854–868 (2019).
Tan, M. H. et al. Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: a scoping review of case reports. Clin. Diabetes Endocrinol. 5, 1 (2019).
Zamami, Y. et al. Factors associated with immune checkpoint inhibitor-related myocarditis. JAMA Oncol. 5, 1635–1637 (2019).
Fenioux, C. et al. Thymus alterations and susceptibility to immune checkpoint inhibitor myocarditis. Nat. Med. 29, 3100–3110 (2023). Describes increased rates of ICI-myocarditis, myositis and myasthenia gravis in patients with thymic epithelial tumors treated with ICI.
Chen, Q. et al. Fatal myocarditis and rhabdomyolysis induced by nivolumab during the treatment of type B3 thymoma. Clin. Toxicol. 56, 667–671 (2018).
Hyun, J.-W. et al. Fatal simultaneous multi-organ failure following pembrolizumab treatment for refractory thymoma. Clin. Lung Cancer 21, e74–e77 (2020).
Nguyen, L. S. et al. Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: proof of concept. J. Immunother. Cancer 10, e004699 (2022).
Yousif, L. I. et al. Risk factors for immune checkpoint inhibitor-mediated cardiovascular toxicities. Curr. Oncol. Rep. 25, 753–763 (2023).
Lyon, A. R., Yousaf, N., Battisti, N. M. L., Moslehi, J. & Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 19, e447–e458 (2018).
Kalinoski, H. et al. Injury-induced myosin-specific tissue-resident memory T cells drive immune checkpoint inhibitor myocarditis. Proc. Natl Acad. Sci. USA 121, e2323052121 (2024).
Pathak, R. et al. Immune checkpoint inhibitor-induced myocarditis with myositis/myasthenia gravis overlap syndrome: a systematic review of cases. Oncologist 26, 1052–1061 (2021).
Aldrich, J. et al. Inflammatory myositis in cancer patients receiving immune checkpoint inhibitors. Arthritis Rheumatol. 73, 866–874 (2021).
Power, J. R. et al. Electrocardiographic manifestations of immune checkpoint inhibitor myocarditis. Circulation 144, 1521–1523 (2021).
Lehmann, L. H. et al. Cardiomuscular biomarkers in the diagnosis and prognostication of immune checkpoint inhibitor myocarditis. Circulation 148, 473–486 (2023).
Pradhan, R., Nautiyal, A. & Singh, S. Diagnosis of immune checkpoint inhibitor-associated myocarditis: a systematic review. Int. J. Cardiol. 296, 113–121 (2019).
Champion, S. N. & Stone, J. R. Immune checkpoint inhibitor associated myocarditis occurs in both high-grade and low-grade forms. Mod. Pathol. 33, 99–108 (2020).
Palaskas, N. L. et al. Immune checkpoint inhibitor myocarditis: elucidating the spectrum of disease through endomyocardial biopsy. Eur. J. Heart Fail. 23, 1725–1735 (2021). Outlines the histopathological features of ICI-myocarditis across a wide range of samples with different disease severity.
Sobol, I., Chen, C. L., Mahmood, S. S. & Borczuk, A. C. Histopathologic characterization of myocarditis associated with immune checkpoint inhibitor therapy. Arch. Pathol. Lab. Med. 144, 1392–1396 (2020).
Ma, P. et al. Expansion of pathogenic cardiac macrophages in immune checkpoint inhibitor myocarditis. Circulation 149, 48–66 (2024). Investigates the role of macrophages in ICI-myocarditis.
Rizzo, S., De Gaspari, M. & Basso, C. Immune checkpoint inhibitor myocarditis: a call for standardized histopathologic criteria. Eur. J. Heart Fail. 23, 1736–1738 (2021).
Norwood, T. G. et al. Smoldering myocarditis following immune checkpoint blockade. J. Immunother. Cancer 5, 91 (2017).
Lehmann, L. H. et al. Clinical strategy for the diagnosis and treatment of immune checkpoint inhibitor-associated myocarditis: a narrative review. JAMA Cardiol. 6, 1329–1337 (2021).
Bonaca, M. P. et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation 140, 80–91 (2019). Provides a standardized criteria for diagnosing ICI-myocarditis based on diagnostic certainty and further classifying based on disease severity.
Moslehi, J. & Salem, J.-E. Immune checkpoint inhibitor myocarditis treatment strategies and future directions. JACC CardioOncol. 4, 704–707 (2022).
Palaskas, N., Lopez‐Mattei, J., Durand, J. B., Iliescu, C. & Deswal, A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J. Am. Heart Assoc. 9, e013757 (2020).
Frascaro, F. et al. Immune checkpoint inhibitors-associated myocarditis: diagnosis, treatment and current status on rechallenge. J. Clin. Med. 12, 7737 (2023).
Thuny, F. et al. Management of immune checkpoint inhibitor-induced myocarditis: the French Working Group’s plea for a pragmatic approach. JACC CardioOncol. 3, 157 (2021).
Wei, S. C. et al. A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov. 11, 614–625 (2021). Developed a mouse model of myocarditis through combined deficiency of PD-1 and CTLA4 in C57BL/6 mice.
Salem, J.-E. et al. Abatacept/ruxolitinib and screening for concomitant respiratory muscle failure to mitigate fatality of immune-checkpoint inhibitor myocarditis. Cancer Discov. 13, 1100–1115 (2023). Describes the use of abatacept and ruxolitinib to prevent mortality in ICI-myocarditis.
Tivol, E. A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995). Studies the effect of CTLA4 deficiency in mouse models.
Waterhouse, P. et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science 270, 985–988 (1995). Also studies the effect of CTLA4 deficiency in mouse models.
Oosterwegel, M. A. et al. The role of CTLA-4 in regulating TH2 differentiation. J. Immunol. 163, 2634–2639 (1999).
Lühder, F., Chambers, C., Allison, J. P., Benoist, C. & Mathis, D. Pinpointing when T cell costimulatory receptor CTLA-4 must be engaged to dampen diabetogenic T cells. Proc. Natl. Acad. Sci. USA 97, 12204–12209 (2000).
Klocke, K., Sakaguchi, S., Holmdahl, R. & Wing, K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc. Natl Acad. Sci. USA 113, E2383–E2392 (2016).
Nishimura, H., Nose, M., Hiai, H., Minato, N. & Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 (1999).
Nishimura, H. et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 (2001). Investigates cardiovascular effects of PD-1 deficiency in mice.
Okazaki, T. et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 9, 1477–1483 (2003).
Wang, J. et al. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc. Natl. Acad. Sci. USA 102, 11823–11828 (2005).
Richard, M. L. & Gilkeson, G. Mouse models of lupus: what they tell us and what they don’t. Lupus Sci. Med. 5, e000199 (2018).
Lucas, J. A. et al. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J. Immunol. 181, 2513–2521 (2008).
Wang, J. et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int. Immunol. 22, 443–452 (2010).
Zhang, Y. et al. Hormonal therapies up-regulate MANF and overcome female susceptibility to immune checkpoint inhibitor myocarditis. Sci. Transl. Med. 14, eabo1981 (2022). Investigates potential drivers of sex differences in the severity of ICI-myocarditis.
Axelrod, M. L. et al. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature 611, 818–826 (2022). Identifies MHC-I-restricted antigenic regions of α-myosin in both human samples and mouse models of ICI-myocarditis.
Okazaki, T. & Honjo, T. The PD-1–PD-L pathway in immunological tolerance. Trends Immunol. 27, 195–201 (2006).
Okazaki, T. et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J. Exp. Med. 208, 395–407 (2011). Describes the effects of combination PD-1 and LAG3 deficiency in mice.
Miyazaki, T., Dierich, A., Benoist, C. & Mathis, D. Independent modes of natural killing distinguished in mice lacking Lag3. Science 272, 405–408 (1996).
Woo, S.-R. et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72, 917–927 (2012).
Michel, L. et al. Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur. Heart J. 43, 316–329 (2022).
Xia, W., Zou, C., Chen, H., Xie, C. & Hou, M. Immune checkpoint inhibitor induces cardiac injury through polarizing macrophages via modulating microRNA-34a/Kruppel-like factor 4 signaling. Cell Death Dis. 11, 575 (2020).
Gergely, T. G. et al. Characterization of immune checkpoint inhibitor-induced cardiotoxicity reveals interleukin-17A as a driver of cardiac dysfunction after anti-PD-1 treatment. Br. J. Pharmacol. 180, 740–761 (2023).
Sun, S.-J. et al. Gasdermin-E-mediated pyroptosis drives immune checkpoint inhibitor-associated myocarditis via cGAS–STING activation. Nat. Commun. 15, 6640 (2024).
Won, T. et al. Cardiac myosin-specific autoimmune T cells contribute to immune-checkpoint-inhibitor-associated myocarditis. Cell Rep. 41, 111611 (2022). Developed a mouse model of ICI-myocarditis and characterized immunopathogenesis in A/J mice treated with anti-PD-1 antibodies.
Massilamany, C., Gangaplara, A., Chapman, N., Rose, N. & Reddy, J. Detection of cardiac myosin heavy chain-α-specific CD4 cells by using MHC class II/IAk tetramers in A/J mice. J. Immunol. Methods 372, 107–118 (2011).
Tam, W. Y. & Cheung, K.-K. Phenotypic characteristics of commonly used inbred mouse strains. J. Mol. Med. 98, 1215–1234 (2020).
Roche, J. A. et al. Myofiber damage precedes macrophage infiltration after in vivo injury in dysferlin-deficient A/J mouse skeletal muscle. Am. J. Pathol. 185, 1686–1698 (2015).
Dillingham, B. C. et al. Inhibition of inflammation with celastrol fails to improve muscle function in dysferlin-deficient A/J mice. J. Neurol. Sci. 356, 157–162 (2015).
Sellers, R. S., Clifford, C. B., Treuting, P. M. & Brayton, C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet. Pathol. 49, 32–43 (2012).
Lee, B. H., Gauna, A. E., Pauley, K. M., Park, Y.-J. & Cha, S. Animal models in autoimmune diseases: lessons learned from mouse models for Sjögren’s syndrome. Clin. Rev. Allergy Immunol. 42, 35–44 (2012).
Hu, Y., Nakagawa, Y., Purushotham, K. R. & Humphreys-Beher, M. G. Functional changes in salivary glands of autoimmune disease-prone NOD mice. Am. J. Physiol. 263, E607–E614 (1992).
Humphreys-Beher, M. G. Animal models for autoimmune disease-associated xerostomia and xerophthalmia. Adv. Dent. Res. 10, 73–75 (1996).
Fontes, J. A. et al. Complete Freund’s adjuvant induces experimental autoimmune myocarditis by enhancing IL-6 production during initiation of the immune response. Immun. Inflamm. Dis. 5, 163–176 (2017).
Čiháková, D., Sharma, R. B., Fairweather, D., Afanasyeva, M. & Rose, N. R. Animal models for autoimmune myocarditis and autoimmune thyroiditis. In Autoimmunity: Methods and Protocols (ed. Perl, A.) 175–193 (Humana Press, 2004).
Neu, N. et al. Cardiac myosin induces myocarditis in genetically predisposed mice. J. Immunol. 139, 3630–3636 (1987).
Pummerer, C. L. et al. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J. Clin. Invest. 97, 2057–2062 (1996).
Valaperti, A. et al. CD11b+ monocytes abrogate TH17 CD4+ T cell-mediated experimental autoimmune myocarditis. J. Immunol. 180, 2686–2695 (2008).
Baldeviano, G. C. et al. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ. Res. 106, 1646–1655 (2010).
Afanasyeva, M., Georgakopoulos, D. & Rose, N. R. Autoimmune myocarditis: cellular mediators of cardiac dysfunction. Autoimmun. Rev. 3, 476–486 (2004).
Afanasyeva, M. et al. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: correlation with cardiac function. Am. J. Pathol. 164, 807–815 (2004).
Rose, N. R., Wolfgram, L. J., Herskowitz, A. & Beisel, K. W. Postinfectious autoimmunity: two distinct phases of Coxsackievirus B3-induced myocarditis. Ann. N. Y. Acad. Sci. 475, 146–156 (1986).
Fairweather, D., Kaya, Z., Shellam, G. R., Lawson, C. M. & Rose, N. R. From infection to autoimmunity. J. Autoimmun. 16, 175–186 (2001).
Fairweather, D. & Rose, N. R. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods 41, 118–122 (2007).
do Canto Cavalheiro, M. M. & Leon, L. L. In Handbook of Animal Models of Infection (eds Zak, O. & Sande, M. A.) 801–810 (Academic, 1999).
Grabie, N. et al. IL-12 is required for differentiation of pathogenic CD8+ T cell effectors that cause myocarditis. J. Clin. Invest. 111, 671–680 (2003).
Tarrio, M. L., Grabie, N., Bu, D., Sharpe, A. H. & Lichtman, A. H. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J. Immunol. 188, 4876–4884 (2012).
Van der Borght, K. et al. Myocarditis elicits dendritic cell and monocyte infiltration in the heart and self-antigen presentation by conventional type 2 dendritic cells. Front. Immunol. 9, 2714 (2018).
Eriksson, U. et al. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat. Med. 9, 1484–1490 (2003).
Machino‐Ohtsuka, T. et al. Tenascin‐C aggravates autoimmune myocarditis via dendritic cell activation and TH17 cell differentiation. J. Am. Heart Assoc. 3, e001052 (2014).
Blyszczuk, P. et al. Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy. Circ. Res. 105, 912–920 (2009).
Tsuruoka, K. et al. Exacerbation of autoimmune myocarditis by an immune checkpoint inhibitor is dependent on its time of administration in mice. Int. J. Cardiol. 313, 67–75 (2020).
Seko, Y., Yagita, H., Okumura, K., Azuma, M. & Nagai, R. Roles of programmed death-1 (PD-1)/PD-1 ligands pathway in the development of murine acute myocarditis caused by coxsackievirus B3. Cardiovasc. Res. 75, 158–167 (2007).
Machado, F. S. et al. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin. Immunopathol. 34, 753–770 (2012).
Gutierrez, F. R. S. et al. Regulation of Trypanosoma cruzi-induced myocarditis by programmed death cell receptor 1. Infect. Immun. 79, 1873–1881 (2011).
Engman, D. M., Dragon, E. A. & Donelson, J. E. Human humoral immunity to hsp70 during Trypanosoma cruzi infection. J. Immunol. 144, 3987–3991 (1990).
Levin, M. J. et al. Identification of major Trypanosoma cruzi antigenic determinants in chronic Chagas’ heart disease. Am. J. Trop. Med. Hyg. 41, 530–538 (1989).
Van Voorhis, W. C. & Eisen, H. Fl-160. A surface antigen of Trypanosoma cruzi that mimics mammalian nervous tissue. J. Exp. Med. 169, 641–652 (1989).
Taqueti, V. R. et al. T-bet controls pathogenicity of CTLs in the heart by separable effects on migration and effector activity. J. Immunol. 177, 5890–5901 (2006).
Grabie, N. et al. Neutrophils sustain pathogenic CD8+ T cell responses in the heart. Am. J. Pathol. 163, 2413–2420 (2003).
Grabie, N. et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell-mediated injury in the heart. Circulation 116, 2062–2071 (2007).
Marty, R. R. et al. MyD88 signaling controls autoimmune myocarditis induction. Circulation 113, 258–265 (2006).
Horiguchi, H. et al. ANGPTL2 promotes immune checkpoint inhibitor-related murine autoimmune myocarditis. Commun. Biol. 6, 965 (2023). Outlines a role for inflammatory fibroblasts in the pathogenesis of ICI-myocarditis.
Du, S. et al. PD-1 modulates radiation-induced cardiac toxicity through cytotoxic T lymphocytes. J. Thorac. Oncol. 13, 510–520 (2018).
Hayashi, T., Lim, K. R. Q., Kovacs, A. & Mann, D. L. Recurrent adrenergic stress provokes persistent myocarditis in PD-1-deficient mice. JACC Basic Transl. Sci. 8, 1503–1517 (2023).
Rubio‐Infante, N. et al. Previous cardiovascular injury is a prerequisite for immune checkpoint inhibitor‐associated lethal myocarditis in mice. ESC Heart Fail. 11, 1249–1257 (2023).
Varga, Z. et al. Prior cardiac ischemic injury exacerbates immune checkpoint inhibitor-induced cardiotoxicity. Cardiovasc. Res. 120, cvae088.091 (2024).
Dendrou, C. A., Petersen, J., Rossjohn, J. & Fugger, L. HLA variation and disease. Nat. Rev. Immunol. 18, 325–339 (2018).
Hahn, M., Nicholson, M. J., Pyrdol, J. & Wucherpfennig, K. W. Unconventional topology of self peptide–major histocompatibility complex binding by a human autoimmune T cell receptor. Nat. Immunol. 6, 490–496 (2005).
Lang, H. L. E. et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat. Immunol. 3, 940–943 (2002).
Sethi, D. K. et al. A highly tilted binding mode by a self-reactive T cell receptor results in altered engagement of peptide and MHC. J. Exp. Med. 208, 91–102 (2011).
Yang, J. et al. Autoreactive T cells specific for insulin B:11–23 recognize a low-affinity peptide register in human subjects with autoimmune diabetes. Proc. Natl Acad. Sci. USA 111, 14840–14845 (2014).
Jensen, P. E. & Zhou, Z. Structural characteristics of HLA-DQ that may impact DM editing and susceptibility to type-1 diabetes. Front. Immunol. 4, 262 (2013).
McKenna, C. J., Codd, M. B., McCann, H. A. & Sugrue, D. D. Idiopathic dilated cardiomyopathy: familial prevalence and HLA distribution. Heart 77, 549–552 (1997).
Limas, C. J. Autoimmunity in dilated cardiomyopathy and the major histocompatibility complex. Int. J. Cardiol. 54, 113–116 (1996).
Liu, J., Purdy, L. E., Rabinovitch, S., Jevnikar, A. M. & Elliott, J. F. Major DQ8-restricted T-cell epitopes for human GAD65 mapped using human CD4, DQA1*0301, DQB1*0302 transgenic IA(null) NOD mice. Diabetes 48, 469–477 (1999).
Elliott, J. F. et al. Autoimmune cardiomyopathy and heart block develop spontaneously in HLA-DQ8 transgenic IAβ knockout NOD mice. Proc. Natl Acad. Sci. USA 100, 13447–13452 (2003).
Taneja, V. et al. Spontaneous myocarditis mimicking human disease occurs in the presence of an appropriate MHC and non-MHC background in transgenic mice. J. Mol. Cell. Cardiol. 42, 1054–1064 (2007).
Taylor, J. A. et al. A spontaneous model for autoimmune myocarditis using the human MHC molecule HLA-DQ81. J. Immunol. 172, 2651–2658 (2004).
Racine, J. J. et al. HLA-DQ8 supports development of insulitis mediated by insulin-reactive human TCR-transgenic T cells in nonobese diabetic mice. J. Immunol. 211, 1792–1805 (2023).
Racine, J. J. et al. Murine MHC-deficient nonobese diabetic mice carrying human HLA-DQ8 develop severe myocarditis and myositis in response to anti-PD-1 immune checkpoint inhibitor cancer therapy. J. Immunol. 212, 1287–1306 (2024).
Hayward, S. L. et al. CD4 T cells play major effector role and CD8 T cells initiating role in spontaneous autoimmune myocarditis of HLA-DQ8 transgenic IAb knockout nonobese diabetic mice. J. Immunol. 176, 7715–7725 (2006).
Čiháková, D. T cells and macrophages drive pathogenesis of immune checkpoint inhibitor myocarditis. Circulation 149, 67–69 (2024).
Lv, H. et al. Impaired thymic tolerance to α-myosin directs autoimmunity to the heart in mice and humans. J. Clin. Invest. 121, 1561–1573 (2011). Shows how lack of expression of α-myosin in the thymus leads to a breach of central tolerance, which drives myocarditis. Enforced expression of α-myosin in the thymus mitigated the development of myocarditis.
Weiss, A. & Leinwand, L. A. The mammalian myosin heavy chain gene family. Annu. Rev. Cell Dev. Biol. 12, 417–439 (1996).
Lompré, A. M., Nadal-Ginard, B. & Mahdavi, V. Expression of the cardiac ventricular α- and β-myosin heavy chain genes is developmentally and hormonally regulated. J. Biol. Chem. 259, 6437–6446 (1984).
Lompre, A. M. et al. Species- and age-dependent changes in the relative amounts of cardiac myosin isoenzymes in mammals. Dev. Biol. 84, 286–290 (1981).
Clark, W. A., Chizzonite, R. A., Everett, A. W., Rabinowitz, M. & Zak, R. Species correlations between cardiac isomyosins. A comparison of electrophoretic and immunological properties. J. Biol. Chem. 257, 5449–5454 (1982).
Miyata, S., Minobe, W., Bristow, M. R. & Leinwand, L. A. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ. Res. 86, 386–390 (2000).
Blum, S. M. et al. Immune responses in checkpoint myocarditis across heart, blood and tumour. Nature 636, 215–223 (2024).
Li, Y., Heuser, J. S., Cunningham, L. C., Kosanke, S. D. & Cunningham, M. W. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J. Immunol. 177, 8234–8240 (2006).
Stavrakis, S. et al. Activating autoantibodies to the β-1 adrenergic and M2 muscarinic receptors facilitate atrial fibrillation in patients with Graves’ hyperthyroidism. J. Am. Coll. Cardiol. 54, 1309–1316 (2009).
Mascaro-Blanco, A. et al. Consequences of unlocking the cardiac myosin molecule in human myocarditis and cardiomyopathies. Autoimmunity 41, 442–453 (2008).
Kohlgruber, A. C. et al. High-throughput discovery of MHC class I- and II-restricted T cell epitopes using synthetic cellular circuits. Nat. Biotechnol. 43, 623–634 (2024).
Ashby, K. M. & Hogquist, K. A. A guide to thymic selection of T cells. Nat. Rev. Immunol. 24, 103–117 (2024).
Racine, J. J. et al. Improved murine MHC-deficient HLA transgenic NOD mouse models for type 1 diabetes therapy development. Diabetes 67, 923–935 (2018).
Murphy, E. Estrogen signaling and cardiovascular disease. Circ. Res. 109, 687–696 (2011).
Gourdie, R. G., Dimmeler, S. & Kohl, P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat. Rev. Drug Discov. 15, 620–638 (2016).
Cai, K. et al. Programmed death of cardiomyocytes in cardiovascular disease and new therapeutic approaches. Pharmacol. Res. 206, 107281 (2024).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Berry, C. J. et al. Effects of deep sedation or general anesthesia on cardiac function in mice undergoing cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 11, 16 (2009).
Acknowledgements
We acknowledge the following funding sources that made this work possible: AHA predoctoral fellowship award ID 25PRE1375723 (https://doi.org/10.58275/AHA.25PRE1375723.pc.gr.227181), Vanderbilt Medical Scientist Training Program T32 training grant, the NIGMS of the National Institutes of Health (award number T32GM007347), NIH 5R01HL156021-04 (Immunologic and antigenic drivers of immune checkpoint inhibitor-associated myocarditis), NIH 5R01CA227481-05 (Patient- and tumor-specific biomarkers and mechanisms that predict irAEs resulting from checkpoint inhibition), 5P30CA068485-29 (Breast Cancer Research Program), NIH 5R01HL155990-04 (Long-term cardiovascular sequelae of cancer immunotherapies), NIH 5R01HL141466-05 (Novel mechanisms and predictors of VEGF receptor inhibitor-associated hypertension), 5P30CA068485-29 (Translational research and interventional oncology research Program), NIH P01 HL141084 and NIH R01 HL160688.
Author information
Authors and Affiliations
Contributions
R.G.F. performed conceptualization, writing (original draft), visualization, review and editing. J.M.B., J.J.M. and D.B.J. performed content oversight, guidance, review and editing.
Corresponding author
Ethics declarations
Competing interests
R.G.F. has no conflicts of interest to disclose. J.J.M. has consulting or advisory roles for Pfizer, Novartis, Bristol Myers Squibb, Deciphera, Takeda, AstraZeneca, Regeneron, Myovant, Silverback Therapeutics, Kurome Therapeutics, Kiniksa Pharmaceuticals, Daiichi Sankyo, BeiGene, IQVIA, AskBio, Labcorp, Paladin, Bitterroot Bio, Repare Therapeutics and Cytokinetics. J.J.M. is a co-inventor of a patent related to the use of abatacept in the treatment of ICI-myocarditis. D.B.J. has served on advisory boards or as a consultant for AstraZeneca, BMS, Jackson Laboratory, Merck, Novartis, Pfizer, Targovax and Teiko and has received research funding from BMS and Incyte. J.M.B. reports grants from the NIH/NCI, the DOD, Susan G. Komen and the BCRF during the conduct of the study. J.M.B. also reports grants from Genentech and Incyte and personal fees from AstraZeneca and Eli Lilly outside the submitted work; in addition, J.M.B. has a patent for MHC-I and MHC-II expression to predict immunotherapy outcomes issued.
Peer review
Peer review information
Nature Cardiovascular Research thanks Marinos Kallikourdis, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fankhauser, R.G., Johnson, D.B., Moslehi, J.J. et al. Preclinical mouse models of immune checkpoint inhibitor-associated myocarditis. Nat Cardiovasc Res 4, 526–538 (2025). https://doi.org/10.1038/s44161-025-00640-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44161-025-00640-2