Abstract
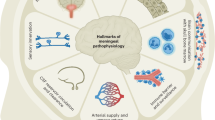
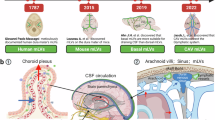
The meninges, consisting of the dura, arachnoid and pia mater that surround the brain and spinal cord, have been recognized from the earliest anatomical studies. First identified in 1787, lymphatic vessels in the dura are now receiving greater attention as their contribution to cerebrospinal fluid (CSF) clearance in diverse neurological conditions is being investigated. New methods have increased the understanding of dural lymphatics, but much is still being learned about their heterogeneity, intracranial and extracranial connections, and factors that govern their functions and maintenance. Current research is striving to understand the regulation of CSF drainage and influence of brain antigen and immune cell transit through dural lymphatics on aging impairments and the severity of neurodegenerative and neuroimmune diseases, traumatic brain injury, stroke and other neurological disorders. Achieving these goals should lead to safe and effective methods for manipulating CSF clearance through dural lymphatics for therapeutic benefit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Proulx, S. T. Cerebrospinal fluid outflow: a review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell. Mol. Life Sci. 78, 2429–2457 (2021).
Betsholtz, C. et al. Advances and controversies in meningeal biology. Nat. Neurosci. 27, 2056–2072 (2024).
Radoš, M., Živko, M., Periša, A., Orešković, D. & Klarica, M. No arachnoid granulations—no problems: number, size, and distribution of arachnoid granulations from birth to 80 years of age. Front. Aging Neurosci. 13, 698865 (2021).
Brierley, J. B. & Field, E. J. The connexions of the spinal sub-arachnoid space with the lymphatic system. J. Anat. 82, 153–166 (1948).
Bradbury, M. S. Lymphatics and the central nervous system. Trends Neurosci. 4, 100–101 (1981).
Cserr, H. F., Harling-Berg, C. J. & Knopf, P. M. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 2, 269–276 (1992).
Johnston, M., Zakharov, A., Papaiconomou, C., Salmasi, G. & Armstrong, D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 1, 2 (2004). By infusing Microfil silicone rubber as a tracer, this study documents the anatomical route from the subarachnoid space through the cribriform plate to the nasal lymphatics in human and non-human primates.
Koh, L., Zakharov, A. & Johnston, M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2, 6 (2005).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015). This study uses confocal and multiphoton microscopy to describe CSF tracer uptake in dural lymphatics located along venous sinuses in the meninges over the dorsal surface of the mouse cerebral cortex after immunofluorescence staining for LYVE1, podoplanin, VEGFR3 and PROX1 as LEC markers.
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015). This study uses Prox1-GFP and Vegfr3+/LacZ reporter mice, immunofluorescence staining of multiple LEC markers and confocal microscopic imaging of whole mounts to characterize dural lymphatic networks near venous sinuses dorsally and near the meningeal vasculature, cribriform plate and other cranial nerve exits basally.
Ma, Q., Ineichen, B. V., Detmar, M. & Proulx, S. T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 8, 1434 (2017). From the results of experiments using Prox1-GFP mice and quantitative methods to monitor CSF tracer outflow to lymph nodes and blood, this study reports that lymphatics are the main route for CSF clearance of small and large molecules from the mouse CNS and that CSF outflow decreases with age.
Louveau, A. et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 21, 1380–1391 (2018). This study reports that dural lymphatics contribute to immune cell trafficking from the CSF to lymph nodes in mice and that the severity of EAE is reduced by ligation of cervical lymphatics that drain to deep cervical lymph nodes.
Ma, Q., Decker, Y., Muller, A., Ineichen, B. V. & Proulx, S. T. Clearance of cerebrospinal fluid from the sacral spine through lymphatic vessels. J. Exp. Med. 216, 2492–2502 (2019).
Ma, Q. et al. Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol. 137, 151–165 (2019).
Spera, I. et al. Open pathways for cerebrospinal fluid outflow at the cribriform plate along the olfactory nerves. EBioMedicine 91, 104558 (2023).
Yoon, J. H. et al. Nasopharyngeal lymphatic plexus is a hub for cerebrospinal fluid drainage. Nature 625, 768–777 (2024). This study uses Prox1-GFP reporter mice and CSF tracers to identify the nasopharyngeal lymphatic plexus as an aging susceptible hub for CSF drainage from dural lymphatics near the cribriform plate and other regions of the skull base to deep cervical lymph nodes.
Jin, H. et al. Increased CSF drainage by noninvasive manipulation of cervical lymphatics. Nature 643, 755–767 (2025). This paper reports that noninvasive mechanical manipulation of superficial cervical lymphatics through the intact skin of the neck by a force-regulated device can increase CSF outflow and correct the impairment of CSF clearance in aged mice.
Proulx, S. T. & Engelhardt, B. Central nervous system zoning: how brain barriers establish subdivisions for CNS immune privilege and immune surveillance. J. Intern. Med. 292, 47–67 (2022).
Pietilä, R. et al. Molecular anatomy of adult mouse leptomeninges. Neuron 111, 3745–3764 (2023).
Smyth, L. C. D. et al. Identification of direct connections between the dura and the brain. Nature 627, 165–173 (2024).
Da Mesquita, S., Fu, Z. & Kipnis, J. The meningeal lymphatic system: a new player in neurophysiology. Neuron 100, 375–388 (2018).
Mapunda, J. A. et al. VE-cadherin in arachnoid and pia mater cells serves as a suitable landmark for in vivo imaging of CNS immune surveillance and inflammation. Nat. Commun. 14, 5837 (2023).
Zanluqui, N. G. & McGavern, D. B. Why do central nervous system barriers host a diverse immune landscape? Trends Immunol. 45, 738–749 (2024).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4, 147ra111 (2012).
Reynolds, W. F., Malle, E. & Maki, R. A. Thiocyanate reduces motor impairment in the hMPO-A53T PD mouse model while reducing MPO-oxidation of alpha synuclein in enlarged LYVE1/AQP4 positive periventricular glymphatic vessels. Antioxidants 11, 2342 (2022).
He, X. Z. et al. High-resolution 3D demonstration of regional heterogeneity in the glymphatic system. J. Cereb. Blood Flow Metab. 42, 2017–2031 (2022).
Meyerhoff, J., Chakraborty, N. & Hammamieh, R. Glymphatics: a transformative development in medical neuroscience relevant to injuries in military central nervous system. Mil. Med. 187, e1086–e1090 (2022).
Reardon, S. Theory of sleep as a brain cleanser challenged. Science 384, 948 (2024).
Albayram, M. S. et al. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat. Commun. 13, 203 (2022).
Chang, J. et al. Characteristic features of deep brain lymphatic vessels and their regulation by chronic stress. Research 6, 0120 (2023).
Li, D. et al. Photostimulation of brain lymphatics in male newborn and adult rodents for therapy of intraventricular hemorrhage. Nat. Commun. 14, 6104 (2023).
Semyachkina-Glushkovskaya, O. et al. Phototherapy of Alzheimer’s disease: photostimulation of brain lymphatics during sleep: a systematic review. Int. J. Mol. Sci. 24, 10946 (2023).
Bartlett, M. J., Erickson, R. P., Hutchinson, E. B., Witte, R. S. & Witte, M. H. Brain lymphatics: rediscovery and new insights into lymphatic involvement in diseases of human brains. Lymphology 57, 27–33 (2024).
Çavdar, S., Altınöz, D., Dilan Demir, T., Ali Gürses, İ. & Özcan, G. Extracranial transport of brain lymphatics via cranial nerve in human. Neurosci. Lett. 827, 137737 (2024).
Zhao, P., Le, Z., Liu, L. & Chen, Y. Therapeutic delivery to the brain via the lymphatic vasculature. Nano Lett. 20, 5415–5420 (2020).
De Angelis, L. C. et al. Brain lymphatic drainage system in fetus and newborn: birth of a new era of exploration. Lymphology 51, 140–147 (2018).
Sun, B. L. et al. Lymphatic drainage system of the brain: a novel target for intervention of neurological diseases. Prog. Neurobiol. 163–164, 118–143 (2018).
Salehpour, F., Khademi, M., Bragin, D. E. & DiDuro, J. O. Photobiomodulation therapy and the glymphatic system: promising applications for augmenting the brain lymphatic drainage system. Int. J. Mol. Sci. 23, 2975 (2022).
Chen, Y., He, X., Cai, J. & Li, Q. Functional aspects of the brain lymphatic drainage system in aging and neurodegenerative diseases. J. Biomed. Res. 38, 206–221 (2024).
Zhu, X. et al. Surgery induces neurocognitive disorder via neuroinflammation and glymphatic dysfunction in middle-aged mice with brain lymphatic drainage impairment. Front. Neurosci. 18, 1426718 (2024).
Semyachkina-Glushkovskaya, O. et al. Transcranial photobiomodulation of clearance of beta-amyloid from the mouse brain: effects on the meningeal lymphatic drainage and blood oxygen saturation of the brain. Adv. Exp. Med. Biol. 1269, 57–61 (2021).
Liu, X. G. et al. Histomorphological analysis of perfusion parameters and CNS lymphatic vessels in mice: an experimental method study. Neuroreport 35, 160–169 (2024).
Shibata-Germanos, S. et al. Structural and functional conservation of non-lumenized lymphatic endothelial cells in the mammalian leptomeninges. Acta Neuropathol. 139, 383–401 (2020).
Møllgård, K. et al. A mesothelium divides the subarachnoid space into functional compartments. Science 379, 84–88 (2023).
Zhao, L. et al. Lymphatic endothelial-like cells promote glioblastoma stem cell growth through cytokine-driven cholesterol metabolism. Nat. Cancer 5, 147–166 (2024).
Mascagni, P. Vasorum Lymphaticorum Corporis Humani Historia et Ichnographia (Ex typographia Pazzini Carli, 1787).
Hendriksen, M. M. Anatomical mercury: changing understandings of quicksilver, blood, and the lymphatic system, 1650–1800. J. Hist. Med. Allied Sci. 70, 516–548 (2015).
Orsini, D., Vannozzi, F. & Aglianò, M. The anatomical world of Paolo Mascagni. Reasoned reading of the anatomy works of his library. Medicina Historica 1, 84–93 (2017).
Lukić, I. K., Gluncić, V., Ivkić, G., Hubenstorf, M. & Marusić, A. Virtual dissection: a lesson from the 18th century. Lancet 362, 2110–2113 (2003).
Weller, R. O., Kida, S. & Zhang, E. T. Pathways of fluid drainage from the brain–morphological aspects and immunological significance in rat and man. Brain Pathol. 2, 277–284 (1992).
Kida, S., Pantazis, A. & Weller, R. O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol. 19, 480–488 (1993).
Löwhagen, P., Johansson, B. B. & Nordborg, C. The nasal route of cerebrospinal fluid drainage in man. A light-microscope study. Neuropathol. Appl. Neurobiol. 20, 543–550 (1994).
Walter, B. A., Valera, V. A., Takahashi, S. & Ushiki, T. The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol. Appl. Neurobiol. 32, 388–396 (2006).
Key, A. & Retzius, G. Studien in der Anatomie des Nervensystems und des Bindegewebes Vol. 1 (Samson & Wallin, 1875).
Weed, L. H. Studies on cerebro-spinal fluid. No. III: the pathways of escape from the subarachnoid spaces with particular reference to the arachnoid villi. J. Med. Res. 31, 51–91 (1914).
Weed, L. H. The cerebrospinal fluid. Physiol. Rev. 2, 171–203 (1922).
Földi, M. et al. New contributions to the anatomical connections of the brain and the lymphatic system. Acta Anat. 64, 498–505 (1966). This paper reports that ligation of lymphatics in the neck dilates upstream lymphatics and enables histological identification of dural lymphatics around the intracranial surface of the jugular foramen and exiting cranial nerves at the skull base.
Földi, M. The brain and the lymphatic system (I). Lymphology 29, 1–9 (1996).
Földi, M. The brain and the lymphatic system (II). Lymphology 29, 10–14 (1996).
Gomez, D. G., Fenstermacher, J. D., Manzo, R. P., Johnson, D. & Potts, D. G. Cerebrospinal fluid absorption in the rabbit: olfactory pathways. Acta Otolaryngol. 100, 429–436 (1985).
Földi, M., Csillik, B. & Zoltán, O. T. Lymphatic drainage of the brain. Experientia 24, 1283–1287 (1968).
Földi, M. Prelymphatic-lymphatic drainage of the brain. Am. Heart J. 93, 121–124 (1977). This report summarizes experiments to determine the striking brain structural and functional abnormality, termed lymphostatic encephalopathy, that results from ligation of cervical lymphatics and reflects the essential role of lymphatics in CSF outflow and brain health.
Antila, S. et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 214, 3645–3667 (2017). Using loss-of-function and gain-of-function approaches, these investigators describe dural lymphatic development and report that dural lymphatics in adult mice are ablated by VEGF-C–VEGFR3 signaling inhibitors and thus require this signaling for survival, but can be expanded by VEGFR3 activation through sustained delivery of VEGF-C.
Ahn, J. H. et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66 (2019). By analyzing the structure, distribution and function of dural lymphatics after infusion of CSF tracers into Prox1-GFP reporter mice, these investigators report that lymphatics in basolateral regions of the skull drain CSF to deep cervical lymph nodes and that this CSF drainage decreases with age.
Hussain, R. et al. Potentiating glymphatic drainage minimizes post-traumatic cerebral oedema. Nature 623, 992–1000 (2023).
Lecco, V. Probable modification of the lymphatic fissures of the walls of the venous sinuses of the dura mater. Arch. Ital. Otol. Rinol. Laringol. 64, 287–296 (1953).
Andres, K. H., von, During, M., Muszynski, K. & Schmidt, R. F. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat. Embryol. 175, 289–301 (1987). Based on TEM observations of the dorsal region of the rat dura mater, these investigators describe the distribution of lymphatics near the sagittal sinus and confluence of sinuses and provide a report of the ultrastructural features of dural lymphatics.
Kato, S., Yasunaga, A. & Uchida, U. Enzyme-histochemical method for identification of lymphatic capillaries. Lymphology 24, 125–129 (1991).
Miura, M., Kato, S. & von Ludinghausen, M. Lymphatic drainage of the cerebrospinal fluid from monkey spinal meninges with special reference to the distribution of the epidural lymphatics. Arch. Histol. Cytol. 61, 277–286 (1998). 5′-nucleotidase histochemistry and carbon particle injections into CSF are used to characterize lymphatics in spinal dural and epidural tissues of monkeys and demonstrate that the lymphatics are most abundant in ‘ink-cuffs’ around nerve exits at intervertebral foramina of the cervical spinal cord.
Gausas, R. E., Daly, T. & Fogt, F. D2-40 expression demonstrates lymphatic vessel characteristics in the dural portion of the optic nerve sheath. Ophthalmic Plast. Reconstr. Surg. 23, 32–36 (2007).
Killer, H. E., Jaggi, G. P., Miller, N. R., Flammer, J. & Meyer, P. Does immunohistochemistry allow easy detection of lymphatics in the optic nerve sheath? J. Histochem. Cytochem. 56, 1087–1092 (2008).
Furukawa, M., Shimoda, H., Kajiwara, T., Kato, S. & Yanagisawa, S. Topographic study on nerve-associated lymphatic vessels in the murine craniofacial region by immunohistochemistry and electron microscopy. Biomed. Res. 29, 289–296 (2008). This study uses LYVE1 immunohistochemistry in combination with TEM and SEM to examine the structure of dural lymphatics near the cribriform plate and crista galli and demonstrates that the lymphatics could be an anatomically direct route for CSF clearance to nasal lymphatics.
Killer, H. E., Laeng, H. R. & Groscurth, P. Lymphatic capillaries in the meninges of the human optic nerve. J. Neuroophthalmol. 19, 222–228 (1999).
Bucchieri, F., Farina, F., Zummo, G. & Cappello, F. Lymphatic vessels of the dura mater: a new discovery? J. Anat. 227, 702–703 (2015).
Mezey, E. & Palkovits, M. Neuroanatomy: forgotten findings of brain lymphatics. Nature 524, 415 (2015).
Witte, M. H. & Bernas, M. J. Re-rediscovery of the brain’s lymphatic system. Lymphology 48, 161–162 (2015).
Erickson, R. P. The re-discovery of dural (meningeal) lymphatics: amnesia or ambition? Lymphology 56, 125–130 (2023).
Izen, R. M., Yamazaki, T., Nishinaka-Arai, Y., Hong, Y. K. & Mukouyama, Y. S. Postnatal development of lymphatic vasculature in the brain meninges. Dev. Dyn. 247, 741–753 (2018).
Jacob, L. et al. Anatomy and function of the vertebral column lymphatic network in mice. Nat. Commun. 10, 4594 (2019).
Da Mesquita, S. et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature 593, 255–260 (2021).
Li, Z. et al. Blockade of VEGFR3 signaling leads to functional impairment of dural lymphatic vessels without affecting autoimmune neuroinflammation. Sci. Immunol. 8, eabq0375 (2023). By inhibiting VEGF-C–VEGFR3 signaling with a VEGFR3-blocking antibody, soluble VEGF-C/VEGF-D trap, or Vegfr3 gene deletion in LECs of adult mice, these investigators report that regression and functional impairment of dural lymphatics have no effect on the severity of EAE as a model of CNS autoimmunity.
Rustenhoven, J. et al. Age-related alterations in meningeal immunity drive impaired CNS lymphatic drainage. J. Exp. Med. 220, e20221929 (2023).
Antila, S. et al. Sustained meningeal lymphatic vessel atrophy or expansion does not alter Alzheimer’s disease-related amyloid pathology. Nat. Cardiovasc. Res. 3, 474–491 (2024). From studies of transgenic mouse models of Alzheimer’s disease, these investigators report that brain amyloid-β load and behavioral phenotypes are unchanged after either regression or hyperplasia of dural lymphatics induced by manipulation of VEGF-C–VEGFR3 signaling, despite the observed effects on CSF tracer drainage to cervical lymph nodes.
Boisserand, L. S. B. et al. VEGF-C prophylaxis favors lymphatic drainage and modulates neuroinflammation in a stroke model. J. Exp. Med. 221, e20221983 (2024).
Baluk, P. & McDonald, D. M. Buttons and zippers: endothelial junctions in lymphatic vessels. Cold Spring. Harb. Perspect. Med. 12, a041178 (2022).
Tammela, T. & Alitalo, K. Lymphangiogenesis: molecular mechanisms and future promise. Cell 140, 460–476 (2010).
Petrova, T. V. et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 21, 4593–4599 (2002).
Aspelund, A., Robciuc, M. R., Karaman, S., Makinen, T. & Alitalo, K. Lymphatic system in cardiovascular medicine. Circ. Res. 118, 515–530 (2016).
Petrova, T. V. & Koh, G. Y. Organ-specific lymphatic vasculature: from development to pathophysiology. J. Exp. Med. 215, 35–49 (2018).
Petrova, T. V. & Koh, G. Y. Biological functions of lymphatic vessels. Science 369, eaax4063 (2020).
Papadopoulos, Z. et al. Differential impact of lymphatic outflow pathways on cerebrospinal fluid homeostasis. J. Exp. Med. 222, e20241752 (2025).
Choi, D. et al. Piezo1 regulates meningeal lymphatic vessel drainage and alleviates excessive CSF accumulation. Nat. Neurosci. 27, 913–926 (2024).
Boulton, M. et al. Drainage of CSF through lymphatic pathways and arachnoid villi in sheep: measurement of 125l-albumin clearance. Neuropathol. Appl. Neurobiol. 22, 325–333 (1996).
Bozanovic-Sosic, R., Mollanji, R. & Johnston, M. G. Spinal and cranial contributions to total cerebrospinal fluid transport. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R909–R916 (2001).
Absinta, M. et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 6, e29738 (2017). Using high-resolution MRI with intravenous gadolinium contrast agent, this study demonstrates a signal for dural lymphatics near the superior sagittal and straight venous sinuses in humans and marmoset monkeys in the same location as dural lymphatics that are visible microscopically after staining for lymphatic reporters.
Decker, Y. et al. Magnetic resonance imaging of cerebrospinal fluid outflow after low-rate lateral ventricle infusion in mice. JCI Insight 7, e150881 (2022).
Gonuguntla, S. & Herz, J. Unraveling the lymphatic system in the spinal cord meninges: a critical element in protecting the central nervous system. Cell. Mol. Life Sci. 80, 366 (2023).
Ringstad, G. & Eide, P. K. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat. Commun. 11, 354 (2020).
Zhou, Y. et al. Impaired peri-olfactory cerebrospinal fluid clearance is associated with ageing, cognitive decline and dyssomnia. EBioMedicine 86, 104381 (2022).
Jackson, R. T., Tigges, J. & Arnold, W. Subarachnoid space of the CNS, nasal mucosa, and lymphatic system. Arch. Otolaryngol. 105, 180–184 (1979).
Da Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191 (2018). VEGF-C-mediated expansion of dural lymphatics in aged mice improves learning and memory and increases CSF drainage to deep cervical lymph nodes, whereas photodynamic ablation of dorsal dural lymphatics increases amyloid-β deposition in the brain and meninges of Alzheimer’s disease model mice.
Jacob, L. et al. Conserved meningeal lymphatic drainage circuits in mice and humans. J. Exp. Med. 219, e20220035 (2022). Using a fluorescent CSF tracer with light-sheet fluorescence microscopy in mice and systemic injection of gadobutrol contrast with MRI in patients, these investigators create detailed three-dimensional maps of dural lymphatic distribution and drainage patterns and report that the functional anatomy of dural lymphatics is conserved in mice and humans.
Ligocki, A. P. et al. Cerebrospinal fluid flow extends to peripheral nerves further unifying the nervous system. Sci. Adv. 10, eadn3259 (2024).
Erlich, S. S., McComb, J. G., Hyman, S. & Weiss, M. H. Ultrastructural morphology of the olfactory pathway for cerebrospinal fluid drainage in the rabbit. J. Neurosurg. 64, 466–473 (1986).
Asgari, M., de Zelicourt, D. A. & Kurtcuoglu, V. Barrier dysfunction or drainage reduction: differentiating causes of CSF protein increase. Fluids Barriers CNS 14, 14 (2017).
Zenker, W., Bankoul, S. & Braun, J. S. Morphological indications for considerable diffuse reabsorption of cerebrospinal fluid in spinal meninges particularly in the areas of meningeal funnels. An electronmicroscopical study including tracing experiments in rats. Anat. Embryol. 189, 243–258 (1994).
Xiang, T. et al. Effects of increased intracranial pressure on cerebrospinal fluid influx, cerebral vascular hemodynamic indexes, and cerebrospinal fluid lymphatic efflux. J. Cereb. Blood Flow Metab. 42, 2287–2302 (2022).
Bradbury, M. W. & Westrop, R. J. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J. Physiol. 339, 519–534 (1983).
Hu, X. et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 30, 229–243 (2020).
Tsai, H. H. et al. Functional investigation of meningeal lymphatic system in experimental intracerebral hemorrhage. Stroke 53, 987–998 (2022).
Li, X. et al. Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system. Nat. Neurosci. 25, 577–587 (2022).
Feng, J. et al. Impaired meningeal lymphatic drainage in Listeria monocytogenes infection. Front. Immunol. 15, 1382971 (2024).
Kim, H., Moore, S. A. & Johnston, M. G. Potential for intranasal drug delivery to alter cerebrospinal fluid outflow via the nasal turbinate lymphatics. Fluids Barriers CNS 11, 4 (2014).
Rustenhoven, J. et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184, 1000–1016 (2021).
Ling, C., Sandor, M. & Fabry, Z. In situ processing and distribution of intracerebrally injected OVA in the CNS. J. Neuroimmunol. 141, 90–98 (2003).
Ling, C., Sandor, M., Suresh, M. & Fabry, Z. Traumatic injury and the presence of antigen differentially contribute to T-cell recruitment in the CNS. J. Neurosci. 26, 731–741 (2006).
Mohammad, M. G. et al. Immune cell trafficking from the brain maintains CNS immune tolerance. J. Clin. Invest. 124, 1228–1241 (2014).
Hochmeister, S. et al. After injection into the striatum, in vitro-differentiated microglia- and bone marrow-derived dendritic cells can leave the central nervous system via the blood stream. Am. J. Pathol. 173, 1669–1681 (2008).
Merlini, A. et al. Distinct roles of the meningeal layers in CNS autoimmunity. Nat. Neurosci. 25, 887–899 (2022).
Hsu, M. et al. Neuroinflammation creates an immune regulatory niche at the meningeal lymphatic vasculature near the cribriform plate. Nat. Immunol. 23, 581–593 (2022).
Dendrou, C. A., Fugger, L. & Friese, M. A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558 (2015).
Engelhardt, B., Comabella, M. & Chan, A. Multiple sclerosis: immunopathological heterogeneity and its implications. Eur. J. Immunol. 52, 869–881 (2022).
Stromnes, I. M. & Goverman, J. M. Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 1, 1810–1819 (2006).
Stromnes, I. M. & Goverman, J. M. Passive induction of experimental allergic encephalomyelitis. Nat. Protoc. 1, 1952–1960 (2006).
Hsu, M. et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun. 10, 229 (2019).
Song, E. et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577, 689–694 (2020).
Tarasoff-Conway, J. M. et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 11, 457–470 (2015).
Elbert, D. L., Patterson, B. W., Lucey, B. P., Benzinger, T. L. S. & Bateman, R. J. Importance of CSF-based Aβ clearance with age in humans increases with declining efficacy of blood–brain barrier/proteolytic pathways. Commun. Biol. 5, 98 (2022).
Gaspar-Silva, F., Trigo, D. & Magalhaes, J. Ageing in the brain: mechanisms and rejuvenating strategies. Cell. Mol. Life Sci. 80, 190 (2023).
Weller, R. O., Djuanda, E., Yow, H. Y. & Carare, R. O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 117, 1–14 (2009).
Weller, R. O. Pathology of cerebrospinal fluid and interstitial fluid of the CNS: significance for Alzheimer disease, prion disorders and multiple sclerosis. J. Neuropathol. Exp. Neurol. 57, 885–894 (1998).
Koh, G. Y. & McDonald, D. M. Meningeal lymphatics can influence stroke outcome. J. Exp. Med. 221, e20232305 (2024).
Esposito, E. et al. Brain-to-cervical lymph node signaling after stroke. Nat. Commun. 10, 5306 (2019).
Yanev, P. et al. Impaired meningeal lymphatic vessel development worsens stroke outcome. J. Cereb. Blood Flow Metab. 40, 263–275 (2020).
Keuters, M. H. et al. The impact of VEGF-C-induced dural lymphatic vessel growth on ischemic stroke pathology. Transl. Stroke. Res. 16, 781–799 (2024).
Bolte, A. C. et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat. Commun. 11, 4524 (2020).
Ashina, M. Migraine. N. Engl. J. Med. 383, 1866–1876 (2020).
Nelson-Maney, N. P. et al. Meningeal lymphatic CGRP signaling governs pain via cerebrospinal fluid efflux and neuroinflammation in migraine models. J. Clin. Invest. 134, e175616 (2024).
Witten, A., Marotta, D. & Cohen-Gadol, A. Developmental innervation of cranial dura mater and migraine headache: a narrative literature review. Headache 61, 569–575 (2021).
Levy, D. & Moskowitz, M. A. Meningeal mechanisms and the migraine connection. Annu. Rev. Neurosci. 46, 39–58 (2023).
Kuburas, A. & Russo, A. F. Shared and independent roles of CGRP and PACAP in migraine pathophysiology. J. Headache Pain 24, 34 (2023).
Kaag Rasmussen, M. et al. Trigeminal ganglion neurons are directly activated by influx of CSF solutes in a migraine model. Science 385, 80–86 (2024).
Iyengar, S., Johnson, K. W., Ossipov, M. H. & Aurora, S. K. CGRP and the trigeminal system in migraine. Headache 59, 659–681 (2019).
Wiggers, A. et al. Brain barriers and their potential role in migraine pathophysiology. J. Headache Pain 23, 16 (2022).
Weed, L. H. Studies on cerebro-spinal fluid. No. IV: the dual source of cerebro-spinal fluid. J. Med. Res. 31, 93–118 (1914).
Welch, K. & Friedman, V. The cerebrospinal fluid valves. Brain 83, 454–469 (1960).
Shah, T. et al. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J. Exp. Med. 220, e20220618 (2023).
Zakharov, A. et al. Integrating the roles of extracranial lymphatics and intracranial veins in cerebrospinal fluid absorption in sheep. Microvasc. Res. 67, 96–104 (2004).
Pan, W. R., Suami, H., Corlett, R. J. & Ashton, M. W. Lymphatic drainage of the nasal fossae and nasopharynx: preliminary anatomical and radiological study with clinical implications. Head Neck 31, 52–57 (2009).
Favre, J. J., Chaffanjon, P., Passagia, J. G. & Chirossel, J. P. Blood supply of the olfactory nerve. Meningeal relationships and surgical relevance. Surg. Radiol. Anat. 17, 133–138 (1995).
Wang, S. S. et al. Microanatomy and surgical relevance of the olfactory cistern. Microsurgery 28, 65–70 (2008).
Cömert, A. et al. Microsurgical anatomy for intraoperative preservation of the olfactory bulb and tract. J. Craniofac. Surg. 22, 1080–1082 (2011).
Yin, X. et al. Compartmentalized ocular lymphatic system mediates eye-brain immunity. Nature 628, 204–211 (2024).
Földi, M., Csanda, E., Zoltán, O. T. & Dobranovics, I. Oedema of the optic nerve and the retina as a consequence of experimental cervical lymphatic blockage. Angiologica 4, 341–347 (1967).
Uchida, Y. et al. Involvement of claudin-11 in disruption of blood-brain, -spinal cord, and -arachnoid barriers in multiple sclerosis. Mol. Neurobiol. 56, 2039–2056 (2019).
Takeuchi, H. et al. Regional differences in the absolute abundance of transporters, receptors and tight junction molecules at the blood-arachnoid barrier and blood-spinal cord barrier among cervical, thoracic and lumbar spines in dogs. Pharm. Res. 39, 1393–1413 (2022).
Du, T. et al. Restoration of cervical lymphatic vessel function in aging rescues cerebrospinal fluid drainage. Nat. Aging 4, 1418–1431 (2024).
Fournier, A. P. et al. Reduced spinal cord parenchymal cerebrospinal fluid circulation in experimental autoimmune encephalomyelitis. J. Cereb. Blood Flow Metab. 39, 1258–1265 (2019).
Xin, L. et al. Impairment of spinal CSF flow precedes immune cell infiltration in an active EAE model. J. Neuroinflammation 21, 272 (2024).
das Neves, S. P. et al. Meningeal lymphatic function promotes oligodendrocyte survival and brain myelination. Immunity 57, 2328–2343 (2024).
Chung, A. S. & Ferrara, N. Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Biol. 27, 563–584 (2011).
Kirkin, V. et al. MAZ51, an indolinone that inhibits endothelial cell and tumor cell growth in vitro, suppresses tumor growth in vivo. Int. J. Cancer 112, 986–993 (2004).
van Zwam, M. et al. Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J. Mol. Med. 87, 273–286 (2009).
van Zwam, M. et al. Surgical excision of CNS-draining lymph nodes reduces relapse severity in chronic-relapsing experimental autoimmune encephalomyelitis. J. Pathol. 217, 543–551 (2009).
Lynch, D. H. et al. Systemic immunosuppression induced by photodynamic therapy (PDT) is adoptively transferred by macrophages. Photochem. Photobiol. 49, 453–458 (1989).
Tammela, T. et al. Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci. Transl. Med. 3, 69ra11 (2011).
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P. & Malik, A. B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20, 1126–1167 (2014).
Chen, J. et al. Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage. Nat. Commun. 11, 3159 (2020).
Madarasz, A., Xin, L. & Proulx, S. T. Clearance of erythrocytes from the subarachnoid space through cribriform plate lymphatics in female mice. EBioMedicine 107, 105295 (2024).
Mor, D. E. et al. Dopamine induces soluble α-synuclein oligomers and nigrostriatal degeneration. Nat. Neurosci. 20, 1560–1568 (2017).
Zou, W. et al. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Transl Neurodegener 8, 7 (2019).
Ding, X. -B. et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat. Med. 27, 411–418 (2021).
Haley, M. J. et al. Lymphatic network drainage resolves cerebral edema and facilitates recovery from experimental cerebral malaria. Cell Rep. 43, 114217 (2024).
Kovacs, M. A. et al. Meningeal lymphatic drainage promotes T cell responses against Toxoplasma gondii but is dispensable for parasite control in the brain. Elife 11, e80775 (2022).
Ang, P. S., Matrongolo, M. J. & Tischfield, M. A. The growth and expansion of meningeal lymphatic networks are affected in craniosynostosis. Development 149, dev200065 (2022).
Matrongolo, M. J. et al. Piezo1 agonist restores meningeal lymphatic vessels, drainage, and brain-CSF perfusion in craniosynostosis and aged mice. J. Clin. Invest. 134, e171468 (2023).
Hominick, D. et al. VEGF-C promotes the development of lymphatics in bone and bone loss. Elife 7, e34323 (2018).
Koh, L. et al. Development of cerebrospinal fluid absorption sites in the pig and rat: connections between the subarachnoid space and lymphatic vessels in the olfactory turbinates. Anat. Embryol. 211, 335–344 (2006).
Acknowledgements
This work was supported in part by funding from the National Heart, Lung, and Blood Institute grants (R01 HL143896, R01 HL059157 and R01 HL127402, to D.M.D.) from the US National Institutes of Health; Republic of Korea Ministry of Science and Information and Communication Technology to the Institute Basic Science (IBS-R025-D1-2015, to G.Y.K.); Wihuri Foundation, the European Union Horizon 2020 research and innovation program (874708, Theralymph), Sigrid Jusélius Foundation, Cancer Foundation Finland, and the Hospital District of Helsinki and Uusimaa (HUS Diagnostic Center, TYH2022202; to K.A.); Swedish Research Council (2015-00550, to C.B.); Swedish Cancer Society (2018/449, 2018/1154 and 211714Pj, to C.B.); Knut and Alice Wallenberg Foundation (2020.0057, to C.B.); Swedish Brain Foundation (ALZ2019-0130 and ALZ2022-0005, to C.B.); Erling-Persson Family Foundation (to C.B.); Leducq Foundation (22CVD01 and 23CVD02, to C.B.); Fidelity Bermuda Foundation (to S.P. and B.E.); Swiss National Science Foundation (310030_189080, to B.E.; 310030_189226 and 320030_231973, to S.P.; and CRSII5_213535, to S.P. and B.E.); and a National Institute of Neurological Disorders and Stroke grant (R01 NS098273, to J.S.) from the US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
All authors conceived the theme, scope and structure of the Review and collaborated in writing the manuscript and creating the figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McDonald, D.M., Alitalo, K., Betsholtz, C. et al. Cerebrospinal fluid draining lymphatics in health and disease: advances and controversies. Nat Cardiovasc Res 4, 1047–1065 (2025). https://doi.org/10.1038/s44161-025-00705-2
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44161-025-00705-2