Abstract
Depression and anxiety are prevalent in people with a chronic physical illness. Increasing evidence suggests that co-occurring physical and mental illness is associated with shared biological pathways. However, little is known about the brain’s role in mediating links between physical and mental health. Here, using multimodal brain imaging and organ-specific physiological markers from the UK Biobank, we establish prospective associations between the baseline health of seven organs including cardiovascular, pulmonary, musculoskeletal, immune, renal, hepatic and metabolic systems, and mental health outcomes at 4–14 years’ follow-up, focusing on depression and anxiety. We reveal multiple pathways, mediated by the brain, through which poor organ health may lead to poor mental health. We identify lifestyle and environmental factors, including exercise, sedentary behavior, diet, sleep quality, smoking, alcohol intake, education and socioeconomic status that influence mental health through their selective impact on the physiology of specific organ systems and brain structure. Our work reveals the interplay between brain, body and lifestyle, and their collective influence on mental health. Pathways elucidated here may inform behavioral interventions to mitigate or prevent the synergistic co-occurrence of physical and mental disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data were obtained from the UK Biobank. Researchers can register to access all data used in this study via the UK Biobank Access Management System (https://bbams.ndph.ox.ac.uk/ams/).
Code availability
MATLAB code (R2022b, MathWorks) for computing organ health scores is available on GitHub (https://github.com/yetianmed/OrganHealthScore). SEM was performed using the lavaan package (v.0.6-16) in R. GAMLSS was performed using the gamlss package (v.5.4-3) in R. Connectome Workbench (v.2.0.0) was used to visualize brain images.
References
Brandl, F. et al. Common and specific large-scale brain changes in major depressive disorder, anxiety disorders, and chronic pain: a transdiagnostic multimodal meta-analysis of structural and functional MRI studies. Neuropsychopharmacology 47, 1071–1080 (2022).
Goodkind, M. et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315 (2015).
Hettema, J. M. What is the genetic relationship between anxiety and depression? Am. J. Med. Genet. C Semin. Med. Genet. 148c, 140–146 (2008).
Kessler, R. C. et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol. Psychiatr. Sci. 24, 210–226 (2015).
Emsley, R. A. et al. Depressive and anxiety symptoms in patients with schizophrenia and schizophreniform disorder. J. Clin. Psychiatry 60, 747–751 (1999).
Spoorthy, M. S. et al. Comorbidity of bipolar and anxiety disorders: an overview of trends in research. World J. Psychiatry 9, 7–29 (2019).
Schirmbeck, F. et al. Impact of comorbid affective disorders on longitudinal clinical outcomes in individuals at ultra-high risk for psychosis. Schizophr. Bull. 48, 100–110 (2021).
Gold, S. M. et al. Comorbid depression in medical diseases. Nat. Rev. Dis. Primers 6, 69 (2020).
Roy-Byrne, P. P. et al. Anxiety disorders and comorbid medical illness. Gen. Hosp. Psychiatry 30, 208–225 (2008).
Moussavi, S. et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 370, 851–858 (2007).
Felez-Nobrega, M. et al. Multimorbidity, depression with anxiety symptoms, and decrements in health in 47 low- and middle-income countries. J. Affect. Disord. 317, 176–184 (2022).
Agustini, B. et al. Patterns of association between depressive symptoms and chronic medical morbidities in older adults. J. Am. Geriatr. Soc. 68, 1834–1841 (2020).
Shinkov, A. et al. Increased prevalence of depression and anxiety among subjects with metabolic syndrome and known type 2 diabetes mellitus - a population-based study. Postgrad. Med. 130, 251–257 (2018).
Ronaldson, A. et al. Associations between physical multimorbidity patterns and common mental health disorders in middle-aged adults: A prospective analysis using data from the UK Biobank. Lancet Reg. Health Eur. 8, 100149 (2021).
Seo, J. et al. The relationship between multiple chronic diseases and depressive symptoms among middle-aged and elderly populations: results of a 2009 korean community health survey of 156,747 participants. BMC Public Health 17, 844 (2017).
Mulugeta, A. et al. Association between major depressive disorder and multiple disease outcomes: a phenome-wide Mendelian randomisation study in the UK Biobank. Mol. Psychiatry 25, 1469–1476 (2020).
Purves, K. L. et al. A major role for common genetic variation in anxiety disorders. Mol. Psychiatry 25, 3292–3303 (2020).
Milaneschi, Y. et al. Depression heterogeneity and its biological underpinnings: toward immunometabolic depression. Biol. Psychiatry 88, 369–380 (2020).
Sheng, J. et al. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017, 9724371 (2017).
Berk, M. et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 11, 200 (2013).
Zhao, Y. et al. The brain structure, immunometabolic and genetic mechanisms underlying the association between lifestyle and depression. Nat. Ment. Health 1, 736–750 (2023).
Heijmans, M. et al. The stress of being chronically ill: from disease-specific to task-specific aspects. J. Behav. Med. 27, 255–271 (2004).
Schmaal, L. et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 22, 900–909 (2017).
Harrewijn, A. et al. Cortical and subcortical brain structure in generalized anxiety disorder: findings from 28 research sites in the ENIGMA-Anxiety Working Group. Transl. Psychiatry 11, 502 (2021).
Winter, N. R. et al. Quantifying deviations of brain structure and function in major depressive disorder across neuroimaging modalities. JAMA Psychiatry 79, 879–888 (2022).
van Velzen, L. S. et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol. Psychiatry 25, 1511–1525 (2020).
Mrozek, S. et al. Crosstalk between brain, lung and heart in critical care. Anaesth. Crit. Care Pain Med. 39, 519–530 (2020).
McCracken, C. et al. Multi-organ imaging demonstrates the heart-brain-liver axis in UK Biobank participants. Nat. Commun. 13, 7839 (2022).
Delezie, J. et al. Endocrine crosstalk between skeletal muscle and the brain. Front. Neurol. 9, 698 (2018).
Zhao, B. et al. Heart-brain connections: phenotypic and genetic insights from magnetic resonance images. Science 380, abn6598 (2023).
Guo, B. et al. Causal associations of brain structure with bone mineral density: a large-scale genetic correlation study. Bone Res. 11, 37 (2023).
Takeda, S. et al. Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 305–317 (2002).
Tian, Y. E. et al. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat. Med. 29, 1221–1231 (2023).
Tian, Y. E. et al. Evaluation of brain-body health in individuals with common neuropsychiatric disorders. JAMA Psychiatry 80, 567–576 (2023).
Bosman, R. C. et al. Prevalence and course of subthreshold anxiety disorder in the general population: a three-year follow-up study. J. Affect. Disord. 247, 105–113 (2019).
Rodríguez, M. R. et al. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatry 12, 181 (2012).
Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 19, 1523–1536 (2016).
Rutherford, S. et al. The normative modeling framework for computational psychiatry. Nat. Protoc. 17, 1711–1734 (2022).
Dutt, R. K. et al. Mental health in the UK Biobank: a roadmap to self-report measures and neuroimaging correlates. Hum. Brain Mapp. 43, 816–832 (2022).
Davis, K. A. S. et al. Mental health in UK Biobank – development, implementation and results from an online questionnaire completed by 157 366 participants: a reanalysis. BJPsych Open 6, e18 (2020).
Hettema, J. M. et al. A population-based twin study of the relationship between neuroticism and internalizing disorders. Am. J. Psychiatry 163, 857–864 (2006).
Widiger, T. A. et al. Neuroticism is a fundamental domain of personality with enormous public health implications. World Psychiatry 16, 144–145 (2017).
Ohrnberger, J. et al. The dynamics of physical and mental health in the older population. J. Econ. Ageing 9, 52–62 (2017).
Momen, N. C. et al. Association between mental disorders and subsequent medical conditions. N. Engl. J. Med. 382, 1721–1731 (2020).
Murray, C. J. L. et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1223–1249 (2020).
Firth, J. et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry 19, 360–380 (2020).
Penninx, B. W. et al. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 11, 129 (2013).
Grieve, S. M. et al. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 3, 332–339 (2013).
Gates, J. et al. Mental health starts with physical health: current status and future directions of non-pharmacological interventions to improve physical health in first-episode psychosis. Lancet Psychiatry 2, 726–742 (2015).
Firth, J. et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 6, 675–712 (2019).
Rigby, R. A. et al. Generalized additive models for location, scale and shape. J. R. Stat. Soc. Ser. C Appl. Stat. 54, 507–554 (2005).
Marquand, A. F. et al. Conceptualizing mental disorders as deviations from normative functioning. Mol. Psychiatry 24, 1415–1424 (2019).
Bethlehem, R. A. I. et al. Brain charts for the human lifespan. Nature 604, 525–533 (2022).
Borghi, E. et al. Construction of the World Health Organization child growth standards: selection of methods for attained growth curves. Stat. Med. 25, 247–265 (2006).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
Mori, S. et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40, 570–582 (2008).
Alfaro-Almagro, F. et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. NeuroImage 166, 400–424 (2018).
Brain Imaging Documentation (UK Biobank, 2024); https://biobank.ctsu.ox.ac.uk/showcase/showcase/docs/brain_mri.pdf
Eysenck, H. J. et al. Manual of the Eysenck Personality Questionnaire: (EPQ-R Adult) (EdITS/Educational and Industrial Testing Service, 1994).
Rosseel, Y. lavaan: an R package for structural equation modeling. J. Stat. Software https://doi.org/10.18637/jss.v048.i02 (2012).
Kline, R. B. Principles and Practice of Structural Equation Modeling (Guilford Press, 2015).
Byrne, B. M. Structural Equation Modeling with EQS and EQS/Windows (Sage Publications, 1994).
Shan, Z. et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 38, 529–537 (2015).
Smith, R. W. et al. Social isolation and risk of heart disease and stroke: analysis of two large UK prospective studies. Lancet Public Health 6, e232–e239 (2021).
Acknowledgements
This research was conducted using data from UK Biobank (https://www.ukbiobank.ac.uk/), a major biomedical database. We thank the UK Biobank for making the data available and all study participants for generously donating their time to make this resource possible. Y.E.T. was supported by a Mary Lugton Postdoctoral Fellowship and a National Health and Medical Research Council Investigator Grant (APP2026413). A.Z. was supported by a Senior Rebecca L. Cooper Fellowship. E.T.B. was supported by a senior investigator award from the National Institute of Health Research, UK, and the NIHR Cambridge Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
Y.E.T. and A.Z. conceived the idea and designed the study. Y.E.T. compiled the data, performed the analyses, prepared the visualizations and drafted the paper. J.H.C. and E.T.B. provided critical conceptual input. All authors provided critical feedback and edited the final paper.
Corresponding author
Ethics declarations
Competing interests
E.T.B. has consulted for GlaxoSmithKline, SR One, Sosei Heptares and Boehringer Ingelheim. The other authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Bruno Agustini, Lukas Roll and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Mental health assessment.
The severity of depressive (a), anxiety (b) and neuroticism (c) symptoms in healthy comparison (HC, n = 7,749) individuals and individuals who had a lifetime diagnosis of one of the following 4 mental disorders: schizophrenia (SCZ, n = 67), depression (DEP, n = 9,817), bipolar disorder (BD, n = 592) and generalized anxiety disorder (GAD, n = 2,041). Depressive symptom severity was represented by the total score of the Recent Depressive Symptom (RDS-4). The score was re-scaled so that a score of zero indicates no recent depressive symptoms. Anxiety symptom severity was represented by the total score of the Generalized Anxiety Disorder (GAD-7) scale. Neuroticism was assessed by the total score of the Eysenck Neuroticism (N-12) questionnaire. The bottom and top edges of the boxes indicate the 25th and 75th percentiles of the distribution, respectively. The central line indicates the median. The whiskers extend to the most extreme data points that are not considered outliers (1.5-times the interquartile range).
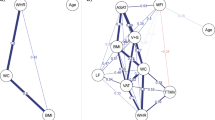
Extended Data Fig. 2 Associations between organ health and mental health.
Prospective associations between baseline organ health of 7 body systems and the severity of depression (a), anxiety (b) and neuroticism (c) symptoms at follow-up. Links are shown for significant associations between the health score of organ systems and mental health measures, adjusting for sex and age at baseline (p < 0.05, two-sided, t-test, false discovery rate (FDR) corrected across 7 organs). Edge thickness reflects standardized regression coefficients (\(\beta\)). Dep, depression; Anx, anxiety; N, neuroticism.
Extended Data Fig. 3 Associations between organ health and mental and brain health.
Prospective associations between baseline organ health of 7 body systems and mental health outcome (a) as well as brain health (b) at follow-up. Links are shown for significant associations between the health score of organ systems and mental/brain health measures, adjusting for sex, age at baseline and individual variation in the time interval between baseline and follow-up assessments (p < 0.05, two-sided, t-test, false discovery rate (FDR) corrected across 7 organs). Edge thickness reflects standardized regression coefficients (\(\beta\)). Dep, depression; Anx, anxiety; N, neuroticism. GMV, total gray matter volume; FA, fractional anisotropy.
Extended Data Fig. 4 Associations between organ health and mental health in patients and healthy individuals.
Prospective associations between baseline organ health of 7 body systems and the severity of depression, anxiety and neuroticism symptoms at follow-up in patients (a) and healthy individuals (HC, b) separately. Links are shown for significant associations between the health score of organ systems and mental health measures, adjusting for sex and age at baseline (p < 0.05, two-sided, t-test, false discovery rate (FDR) corrected across 7 organs). Edge thickness reflects standardized regression coefficients (\(\beta\)). Dep, depression; Anx, anxiety; N, neuroticism.
Extended Data Fig. 5 Mediating effects of gray and white matter on physical-mental health associations.
Pathways linking organ health, brain gray matter volume (a)/ fractional anisotropy (FA) of white matter (b), and mental health. A structural equation model (SEM) linking the health of each organ system to a summary measure of mental health (MH) across depression, anxiety and neuroticism. The summary measure was indicated by the first principal component of the three measures. Links are shown for significant paths linking organ health (exogenous) and mental health (outcome) via brain gray matter volume (a) or white matter tracts (b, mediator) inferred from SEM (p < 0.05, two-sided, false discovery rate corrected for 7 organs). Node size of organs is modulated by the direct effect from organs to mental health outcome. Node size of the brain is modulated by its mediating effect and ranked in decreasing order. Edge thickness reflects regression coefficients estimated for edges comprising the SEM. Non-significant mediating effect of the brain is indicated by a dashed square. Pulmon., pulmonary; Muscle., musculoskeletal; Cardiovas., cardiovascular; Dep, depression; Anx, anxiety; N, neuroticism; n.s., non-significant.
Extended Data Fig. 6 Mediating effects of gray and white matter regions on physical-mental health associations.
A structural equation model (SEM) was fitted for each gray matter region, white matter tract and for each organ system. Significant mediating effects (z-statistic) of brain regions (p < 0.05, two-sided, FDR-corrected for 33 gray and 27 white matter regions) were averaged across 7 organ systems to provide a consensus mediating effect map for overall mental health. The overall mental health (MH) score was indicated by the first principal component across three measures, that is, depression, anxiety and neuroticism. a, The average z-statistic for cortical gray matter volume (Desikan-Killianny atlas) are rendered on cortical surface for visualization. Word clouds show top‐ranked cortical and subcortical regions. The font size is scaled according to the absolute value of the average z-statistic. b, Similarly, the average z-statistic for regional fractional anisotropy (JHU ICBM-DTI-81 atlas) are rendered in standard Montreal Neurological Institute (MNI)-152 anatomical space. Word clouds show top‐ranked white matter tracts. The font size is scaled according to the absolute value of the average z-statistic.
Extended Data Fig. 7 Associations between lifestyle factors and mental health.
Bar plots show the prospective associations between baseline exposure to lifestyle factors and the severity of depression (a), anxiety (b) and neuroticism (c) symptoms at follow-up. Colored bars indicate lifestyle factors with significant associations, adjusting for sex and age at baseline (p < 0.05, two-sided, FDR corrected across 14 lifestyle factors). Lifestyle factors are ordered from top to bottom according to decreasing regression coefficient.
Supplementary information
Supplementary Tables
Supplementary Tables 1–9.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, Y.E., Cole, J.H., Bullmore, E.T. et al. Brain, lifestyle and environmental pathways linking physical and mental health. Nat. Mental Health 2, 1250–1261 (2024). https://doi.org/10.1038/s44220-024-00303-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44220-024-00303-4
This article is cited by
-
Neural correlates of human fear conditioning and sources of variability in 2199 individuals
Nature Communications (2025)