Abstract
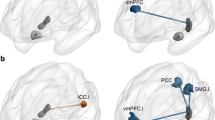
Pattern separation and pattern completion are opposing yet complementary components of mnemonic processing that rely heavily on the hippocampus. It has been shown that processing within the dentate gyrus (DG) subfield promotes pattern separation while operations within the CA3 subfield are important for pattern completion. Schizophrenia has been associated with anatomical and functional hippocampal abnormalities, including within the DG and CA3. We hypothesized that an impairment in hippocampal circuitry in individuals with first-episode schizophrenia leads to deficits in pattern separation (mnemonic discrimination) and pattern completion (recognition memory), that these deficits contribute to delusions and that antipsychotic treatment improves circuit functioning. We measured behavioral and neural responses during the identification of new, repeated and similar stimuli using high-resolution fMRI in 45 medication-free or minimally treated individuals with first-episode schizophrenia (FES) and 49 matched healthy controls (HC). We found recognition memory and pattern separation deficits in FES and a negative association between memory performance and the severity of delusions. Neural analyses revealed deficits in BOLD responses in the hippocampus during mnemonic discrimination in FES compared with HC. Importantly, by investigating the association between trial-level neural activity and behavior before and after treatment, we found that antipsychotics normalized DG activity during pattern separation. Last, trial-level cortical responses during mnemonic discrimination predicted performance in FES at baseline, suggesting a compensatory role. This case-control study provides important insight into the impact of schizophrenia and antipsychotic treatment on memory systems and uncovers systems-level contributions to pattern separation and pattern completion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The dataset collected for this work includes self-reported demographic information, clinical evaluations and neurocognitive and functional neuroimaging data from individuals with first-episode psychosis. The original dataset contained identifying information (required for the process of clinical care), and even after de-identifying the data, there might be a possibility of revelation of the identity of individuals with uncommon characteristics. Thus, the data will be available on request to users who provide a data-sharing agreement that ensures protecting the anonymity of participants through safe handling and storing of data according to our protocol. Data access shall be requested by contacting the corresponding author (ld24@columbia.edu) and will be responded to promptly.
Code availability
Codes to perform the main analyses of the paper are available in the following GitHub repository: https://github.com/azadbood/schizo_hipp_circuit_ps.
References
Aleman, A., Hijman, R., de Haan, E. H. F. & Kahn, R. S. Memory impairment in schizophrenia: a meta-analysis. Am. J. Psychiatry 156, 1358–1366 (1999).
Guo, J., Ragland, J. & Carter, C. Memory and cognition in schizophrenia. Mol. Psychiatry 24, 633–642 (2019).
Avery, S. N. et al. Impaired relational memory in the early stage of psychosis. Schizophr. Res. 212, 113–120 (2019).
Greenland-White, S. E., Ragland, J. D., Niendam, T. A., Ferrer, E. & Carter, C. S. Episodic memory functions in first episode psychosis and clinical high risk individuals. Schizophr. Res. 188, 151–157 (2017).
Huron, C. et al. Impairment of recognition memory with, but not without, conscious recollection in schizophrenia. Am. J. Psychiatry 152, 1737–1742 (1995).
Favre, G. et al. False memory production in schizophrenia: a neurophysiological investigation. Schizophr. Res. Cogn. 20, 100174 (2020).
Bhatt, R., Laws, K. R. & McKenna, P. J. False memory in schizophrenia patients with and without delusions. Psychiatry Res. 178, 260–265 (2010).
Bogerts, B. et al. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. 35, 1–13 (1990).
Kraguljac, N. V., White, D. M., Reid, M. A. & Lahti, A. C. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry 70, 1294–1302 (2013).
McHugo, M. et al. Hippocampal volume in early psychosis: a 2-year longitudinal study. Transl. Psychiatry 10, 306 (2020).
Van Erp, T. G. M. et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry 21, 547–553 (2016).
Ongür, D. et al. The neural basis of relational memory deficits in schizophrenia. Arch. Gen. Psychiatry 63, 356–365 (2006).
Heckers, S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 11, 520–528 (2001).
Heckers, S. et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat. Neurosci. 1, 318–323 (1998).
Achim, A. M. et al. Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Arch. Gen. Psychiatry 64, 999–1014 (2007).
Ragland, J. D. et al. Functional and neuroanatomic specificity of episodic memory dysfunction in schizophrenia: a functional magnetic resonance imaging study of the relational and item-specific encoding task. JAMA Psychiatry 72, 909–916 (2015).
Samudra, N. et al. Alterations in hippocampal connectivity across the psychosis dimension. Psychiatry Res. 233, 148–157 (2015).
Blessing, E. M. et al. Anterior hippocampal–cortical functional connectivity distinguishes antipsychotic naïve first-episode psychosis patients from controls and may predict response to second-generation antipsychotic treatment. Schizophr. Bull. 46, 680–689 (2020).
Kraguljac, N. V. et al. Aberrant hippocampal connectivity in unmedicated patients with schizophrenia and effects of antipsychotic medication: a longitudinal resting state functional MRI study. Schizophr. Bull. 42, 1046–1055 (2016).
Tamminga, C. A., Stan, A. D. & Wagner, A. D. The hippocampal formation in schizophrenia. Am. J. Psychiatry 167, 1178–1193 (2010).
Lieberman, J. A. et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol. Psychiatry 23, 1764–1772 (2018).
Knight, S. et al. Hippocampal circuit dysfunction in psychosis. Transl. Psychiatry 12, 344 (2022).
Tamminga, C. A. Psychosis is emerging as a learning and memory disorder. Neuropsychopharmacology 38, 247 (2013).
Perez, J. M. et al. Hippocampal subfield transcriptome analysis in schizophrenia psychosis. Mol. Psychiatry 26, 2577–2589 (2021).
Tavitian, A., Song, W. & Schipper, H. M. Dentate gyrus immaturity in schizophrenia. Neuroscientist 25, 528–547 (2019).
Li, W. et al. Volume alteration of hippocampal subfields in first-episode antipsychotic-naïve schizophrenia patients before and after acute antipsychotic treatment. Neuroimage Clin. 20, 169–176 (2018).
Li, W. et al. Synaptic proteins in the hippocampus indicative of increased neuronal activity in CA3 in schizophrenia. Am. J. Psychiatry 172, 373–382 (2015).
Hainmueller, T. & Bartos, M. Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat. Rev. Neurosci. 21, 153–168 (2020).
Kesner, R. P. An analysis of dentate gyrus function (an update). Behav. Brain Res. 354, 84–91 (2018).
Clelland, C. D. et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213 (2009).
Youssef, M. et al. Early life stress delays hippocampal development and diminishes the adult stem cell pool in mice. Sci. Rep. 9, 4120 (2019).
Van Erp, T. G. M. et al. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am. J. Psychiatry 159, 1514–1520 (2002).
Miller, B. J. & Goldsmith, D. R. Evaluating the hypothesis that schizophrenia is an inflammatory disorder. Focus 18, 391–401 (2020).
Steullet, P. et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J. Neurosci. 30, 2547–2558 (2010).
Reif, A. et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry 11, 514–522 (2006).
Neunuebel, J. P. & Knierim, J. J. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron 81, 416–427 (2014).
Nakazawa, K. et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297, 211–218 (2002).
Kolomeets, N. S., Orlovskaya, D. D., Rachmanova, V. I. & Uranova, N. A. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse 57, 47–55 (2005).
Kolomeets, N. S., Orlovskaya, D. D. & Uranova, N. A. Decreased numerical density of CA3 hippocampal mossy fiber synapses in schizophrenia. Synapse 61, 615–621 (2007).
Koller, W. N. & Cannon, T. D. Aberrant memory and delusional ideation: a pernicious partnership? Clin. Psychol. Rev. 99, 102231 (2023).
Bodnar, M. et al. The effect of second-generation antipsychotics on hippocampal volume in first episode of psychosis: longitudinal study. BJPsych Open 2, 139–146 (2016).
Pidgeon, L. M. & Morcom, A. M. Cortical pattern separation and item-specific memory encoding. Neuropsychologia 85, 256–271 (2016).
Amer, T. & Davachi, L. Extra-hippocampal contributions to pattern separation. eLife 12, e82250 (2023).
Stark, S. M., Kirwan, C. B. & Stark, C. E. L. Mnemonic Similarity Task: a tool for assessing hippocampal integrity. Trends Cogn. Sci. 23, 938–951 (2019).
Das, T., Ivleva, E. I., Wagner, A. D., Stark, C. E. L. & Tamminga, C. A. Loss of pattern separation performance in schizophrenia suggests dentate gyrus dysfunction. Schizophr. Res. 159, 193–197 (2014).
Martinelli, C. & Shergill, S. S. Clarifying the role of pattern separation in schizophrenia: the role of recognition and visual discrimination deficits. Schizophr. Res. 166, 328–333 (2015).
Kraguljac, N. V. et al. Mnemonic discrimination deficits in first-episode psychosis and a ketamine model suggest dentate gyrus pathology linked to NMDA receptor hypofunction. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6, 1185–1192 (2021).
Chakos, M. H. et al. Duration of illness and treatment effects on hippocampal volume in male patients with schizophrenia. Br. J. Psychiatry 186, 26–31 (2005).
Velakoulis, D. et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch. Gen. Psychiatry 63, 139–149 (2006).
Haddock, G., McCarron, J., Tarrier, N. & Faragher, E. B. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol. Med. 29, 879–889 (1999).
Bakker, A., Kirwan, C. B., Miller, M. & Stark, C. E. L. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 319, 1640–1642 (2008).
Kirwan, C. B. & Stark, C. E. L. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn. Mem. 14, 625–633 (2007).
Leucht, S. et al. Clinical implications of Brief Psychiatric Rating Scale scores. Br. J. Psychiatry 187, 366–371 (2005).
Leucht, S. et al. What does the PANSS mean? Schizophr. Res. 79, 231–238 (2005).
Hu, N. et al. Anatomic abnormalities of hippocampal subfields in never-treated and antipsychotic-treated patients with long-term schizophrenia. Eur. Neuropsychopharmacol. 35, 39–48 (2020).
Pujol, N. et al. Hippocampal abnormalities and age in chronic schizophrenia: morphometric study across the adult lifespan. Br. J. Psychiatry 205, 369–375 (2014).
Pirnia, T. et al. Hippocampal dysfunction during declarative memory encoding in schizophrenia and effects of genetic liability. Schizophr. Res. 161, 357–366 (2015).
Nuechterlein, K. H. et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry. 165, 203–213 (2008).
Esteban, O. et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111 (2019).
Gorgolewski K. et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in Python. Front. Neuroinform. https://doi.org/10.3389/fninf.2011.00013 (2011).
Tustison, N. J. et al. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).
Avants, B. B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008).
Zhang, Y., Brady, M. & Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 20, 45–57 (2001).
Reuter, M., Rosas, H. D. & Fischl, B. Highly accurate inverse consistent registration: a robust approach. Neuroimage 53, 1181–1196 (2010).
Fischl, B. FreeSurfer. Neuroimage 62, 774–781 (2012).
Klein, A. et al. Mindboggling morphometry of human brains. PLoS Comput. Biol. 13, e1005350 (2017).
Greve, D. N. & Fischl, B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48, 63–72 (2009).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002).
Power, J. D. et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 84, 320–341 (2014).
Behzadi, Y., Restom, K., Liau, J. & Liu, T. T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101 (2007).
Bein, O. & Davachi, L. Event integration and temporal differentiation: how hierarchical knowledge emerges in hippocampal subfields through learning. J. Neurosci. 44, e0627232023 (2024).
Shirer, W. R., Ryali, S., Rykhlevskaia, E., Menon, V. & Greicius, M. D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex 22, 158–165 (2012).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Woolrich, M. W., Ripley, B. D., Brady, M. & Smith, S. M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14, 1370–1386 (2001).
Mumford, J. A., Turner, B. O., Ashby, F. G. & Poldrack, R. A. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. Neuroimage 59, 2636–2643 (2012).
Mumford, J. A., Davis, T. & Poldrack, R. A. The impact of study design on pattern estimation for single-trial multivariate pattern analysis. Neuroimage 103, 130–138 (2014).
Haxby, J. V. et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293, 2425–2430 (2001).
Kriegeskorte N., Mur M. & Bandettini P. Representational similarity analysis—connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2 (2008).
Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models v.0.4.7 https://CRAN.R-project.org/package=DHARMa (2024).
Acknowledgments
We thank C. Cather for training of raters, W. Yu for her comments on the analyses and T. Hainmueller for his comments on the manuscript. This work was supported by NIH grant R01MH112733 awarded to D.C.G. and L.D.
Author information
Authors and Affiliations
Contributions
L.D. and D.C.G. and A.Z. conceptualized the study and analyses. A.Z., Y.T., W.S. curated the data. A.Z. performed the formal analyses. Y.T., W.S., H.H., S.Y., J.W., V.P.M., C.G., O.B., L.H., Q.J., T.Z., Y.H. performed the investigation (including task administration and clinical and neuroimaging data collection). G.C. was the project administrator. M.F.G. provided the cognitive battery. L.D. and D.C.G. aquired the funding. J.W., D.C.G., and L.D. supervised the study. A.Z, L.D. and D.C,G. conducted the visualization. A.Z., L.D., and D.C.G. wrote the original manuscript draft. All authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Text and Figures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zadbood, A., Tang, Y., Su, W. et al. Impaired hippocampal circuitry and memory dysfunction in schizophrenia. Nat. Mental Health 3, 332–345 (2025). https://doi.org/10.1038/s44220-024-00376-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44220-024-00376-1