Abstract
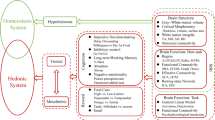
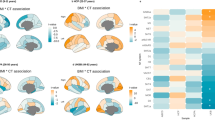
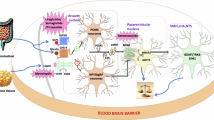
Although obesity has been implicated in brain and cognitive health, the effect of longitudinal obesity trajectories on brain and cognitive aging remains insufficiently understood. Here, using multifaceted obesity measurements from the UK Biobank, we identified five distinct obesity trajectories: low-stable, moderate-stable, high-stable, increasing and decreasing. We observed that individuals in the decreasing trajectory showed minimal adverse effects on brain structure and cognitive performance, compared with the low-stable trajectory (low obesity levels over time). By contrast, the increasing and moderate- and high-stable trajectories were associated with progressively greater impairments in brain morphology, functional connectivity and cognitive abilities. Specifically, adverse effects extended from fronto-mesolimbic regions in the increasing trajectory to parietal and temporal regions in the moderate-stable trajectory, culminating in widespread brain abnormalities in the high-stable group. These findings highlight the dynamic relationship between obesity evolution and brain-cognitive health, underscoring the clinical importance of long-term monitoring and management of obesity through a multifaceted approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The UK Biobank data are a public health database. This work mainly includes demographic data, multiple obesity measures, brain images and cognitive tests. Researchers can apply for access to the data on the UK Biobank website (https://www.ukbiobank.ac.uk). Additional information regarding registration for data access is available at http://www.ukbiobank.ac.uk/register-apply/. The application number for this study is 57831.
Code availability
No new algorithms were written for this study. Study analyses were carried out in MATLAB R2017b (The MathWorks) and Python 3.6. The version of R software is 4.3.1. The code is available from the corresponding author upon reasonable request.
References
World Health Organization. Obesity and overweight, fact sheet. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2024).
Jaacks, L. M. et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 7, 231–240 (2019).
Frank, P. et al. Overweight, obesity, and individual symptoms of depression: a multicohort study with replication in UK Biobank. Brain Behav. Immun. 105, 192–200 (2022).
Avila, C. et al. An overview of links between obesity and mental health. Curr. Obes. Rep. 4, 303–310 (2015).
Tam, B. T., Morais, J. A. & Santosa, S. Obesity and ageing: two sides of the same coin. Obes. Rev. 21, e12991 (2020).
Bischof, G. N. & Park, D. C. Obesity and aging: consequences for cognition, brain structure, and brain function. Psychosom. Med. 77, 697–709 (2015).
Pugazhenthi, S., Qin, L. & Reddy, P. H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 1037–1045 (2017).
Jones, R. A., Lawlor, E. R., Griffin, S. J., van Sluijs, E. M. F. & Ahern, A. L. Impact of adult weight management interventions on mental health: a systematic review and meta-analysis protocol. BMJ Open 10, e031857 (2020).
Payne, M. E. et al. Quality of life and mental health in older adults with obesity and frailty: associations with a weight loss intervention. J. Nutr. Health. Aging 22, 1259–1265 (2018).
Zheng, S. et al. Effectiveness of holistic mobile health interventions on diet, and physical, and mental health outcomes: a systematic review and meta-analysis. EClinicalMedicine 66, 102309 (2023).
Veronese, N. et al. Weight loss is associated with improvements in cognitive function among overweight and obese people: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 72, 87–94 (2017).
Levakov, G. et al. The effect of weight loss following 18 months of lifestyle intervention on brain age assessed with resting-state functional connectivity. eLife 12, e83604 (2023).
Schmitt, L. O. & Gaspar, J. M. Obesity-induced brain neuroinflammatory and mitochondrial changes. Metabolites 13, 86 (2023).
Guillemot-Legris, O. & Muccioli, G. G. Obesity-induced neuroinflammation: beyond the hypothalamus. Trends Neurosci. 40, 237–253 (2017).
Roh, H. T., Cho, S. Y. & So, W. Y. Obesity promotes oxidative stress and exacerbates blood-brain barrier disruption after high-intensity exercise. J. Sport. Health. Sci. 6, 225–230 (2017).
Santos, A. L. & Sinha, S. Obesity and aging: molecular mechanisms and therapeutic approaches. Ageing Res. Rev. 67, 101268 (2021).
Neto, A., Fernandes, A. & Barateiro, A. The complex relationship between obesity and neurodegenerative diseases: an updated review. Front. Cell. Neurosci. 17, 1294420 (2023).
Carnell, S. et al. Neural correlates of familial obesity risk and overweight in adolescence. NeuroImage 159, 236–247 (2017).
Belfort-DeAguiar, R. et al. Humans with obesity have disordered brain responses to food images during physiological hyperglycemia. Am. J. Physiol. Endocrinol. Metab. 314, E522–E529 (2018).
Blechert, J., Klackl, J., Miedl, S. F. & Wilhelm, F. H. To eat or not to eat: effects of food availability on reward system activity during food picture viewing. Appetite 99, 254–261 (2016).
Demos, K. E. et al. The effects of experimental manipulation of sleep duration on neural response to food cues. Sleep 40, zsx125 (2017).
Wiemerslage, L. et al. An obesity-associated risk allele within the FTO gene affects human brain activity for areas important for emotion, impulse control and reward in response to food images. Eur. J. Neurosci. 43, 1173–1180 (2016).
Dodd, S. L., Long, J. D., Hou, J., Kahathuduwa, C. N. & O’Boyle, M. W. Brain activation and affective judgements in response to personal dietary images: an fMRI preliminary study. Appetite 148, 104561 (2020).
Li, G. et al. Brain functional and structural magnetic resonance imaging of obesity and weight loss interventions. Mol. Psychiatry 28, 1466–1479 (2023).
Smith, E., Hay, P., Campbell, L. & Trollor, J. N. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes. Rev. 12, 740–755 (2011).
Wang, C., Chan, J. S., Ren, L. & Yan, J. H. Obesity reduces cognitive and motor functions across the lifespan. Neural. Plast. 2016, 2473081 (2016).
Anstey, K. J., Cherbuin, N., Budge, M. & Young, J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes. Rev. 12, e426–e437 (2011).
Schall, M., Iordanishvili, E., Mauler, J., Oros-Peusquens, A. M. & Shah, N. J. Increasing body mass index in an elderly cohort: effects on the quantitative MR parameters of the brain. J. Magn. Reson. Imaging 51, 514–523 (2020).
Walther, K., Birdsill, A. C., Glisky, E. L. & Ryan, L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum. Brain Mapp. 31, 1052–1064 (2010).
Hamer, M. & Batty, G. D. Association of body mass index and waist-to-hip ratio with brain structure: UK Biobank study. Neurology 92, e594–e600 (2019).
Zhang, Y. et al. Recovery of brain structural abnormalities in morbidly obese patients after bariatric surgery. Int. J. Obes. 40, 1558–1565 (2016).
Almby, K. E. et al. Effects of gastric bypass surgery on the brain: simultaneous assessment of glucose uptake, blood flow, neural activity, and cognitive function during normo- and hypoglycemia. Diabetes 70, 1265–1277 (2021).
Tuulari, J. J. et al. Bariatric surgery induces white and grey matter density recovery in the morbidly obese: a voxel-based morphometric study. Hum. Brain Mapp. 37, 3745–3756 (2016).
Janssen, I. & Mark, A. E. Elevated body mass index and mortality risk in the elderly. Obes. Rev. 8, 41–59 (2007).
Snijder, M. B., van Dam, R. M., Visser, M. & Seidell, J. C. What aspects of body fat are particularly hazardous and how do we measure them? Int. J. Epidemiol. 35, 83–92 (2006).
Kumar, A. et al. Brain–muscle communication prevents muscle aging by maintaining daily physiology. Science 384, 563–572 (2024).
Lee, H. et al. Obesity and muscle may have synergic effect more than independent effects on brain volume in community-based elderly. Eur. Radiol. 31, 2956–2966 (2021).
Wang, R.-Z. et al. Body weight in neurological and psychiatric disorders: a large prospective cohort study. Nat. Ment. Health 2, 41–51 (2024).
Grapsa, I. et al. Longitudinal examination of body mass index and cognitive function in older adults: the HELIAD study. Nutrients 15, 1795 (2023).
Pflanz, C. P. et al. Central obesity is selectively associated with cerebral gray matter atrophy in 15,634 subjects in the UK Biobank. Int. J. Obes. 46, 1059–1067 (2022).
Dekkers, I. A., Jansen, P. R. & Lamb, H. J. Obesity, brain volume, and white matter microstructure at MRI: a cross-sectional UK Biobank study. Radiology 291, 763–771 (2019).
Kim, A. Y., Shim, J. H., Choi, H. J. & Baek, H. M. Comparison of volumetric and shape changes of subcortical structures based on 3-dimensional image between obesity and normal-weighted subjects using 3.0 T MRI. J. Clin. Neurosci. 73, 280–287 (2020).
Franz, C. E. et al. Body mass trajectories and cortical thickness in middle-aged men: a 42-year longitudinal study starting in young adulthood. Neurobiol. Aging 79, 11–21 (2019).
Ambikairajah, A., Tabatabaei-Jafari, H., Walsh, E., Hornberger, M. & Cherbuin, N. Longitudinal changes in fat mass and the hippocampus. Obesity 28, 1263–1269 (2020).
Willette, A. A. & Kapogiannis, D. Does the brain shrink as the waist expands? Ageing Res. Rev. 20, 86–97 (2015).
Driscoll, I. et al. Midlife obesity and trajectories of brain volume changes in older adults. Hum Brain. Mapp. 33, 2204–2210 (2012).
Bobb, J. F., Schwartz, B. S., Davatzikos, C. & Caffo, B. Cross-sectional and longitudinal association of body mass index and brain volume. Hum. Brain Mapp. 35, 75–88 (2014).
Park, B. Y. et al. Whole-brain functional connectivity correlates of obesity phenotypes. Hum. Brain Mapp. 41, 4912–4924 (2020).
Berthoud, H. R., Munzberg, H. & Morrison, C. D. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology 152, 1728–1738 (2017).
Kaplan, J. M., Seeley, R. J. & Grill, H. J. Daily caloric intake in intact and chronic decerebrate rats. Behav. Neurosci. 107, 876–881 (1993).
Friedman, J. M. Leptin and the endocrine control of energy balance. Nat. Metab. 1, 754–764 (2019).
Martín, M. & Ramos, S. Dietary flavonoids and insulin signaling in diabetes and obesity. Cells 10, 1474 (2021).
Steinert, R. E. et al. Ghrelin, CCK, GLP-1, and PYY(3-36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 97, 411–463 (2017).
Low, A. Y. T. et al. Reverse-translational identification of a cerebellar satiation network. Nature 600, 269–273 (2021).
Tregellas, J. R. et al. Altered default network activity in obesity. Obesity 19, 2316–2321 (2011).
McFadden, K. L., Cornier, M. A., Melanson, E. L., Bechtell, J. L. & Tregellas, J. R. Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. NeuroReport 24, 866–871 (2013).
Li, G. et al. Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self-referential processing. Hum. Brain Mapp. 39, 4755–4765 (2018).
Doucet, G. E., Rasgon, N., McEwen, B. S., Micali, N. & Frangou, S. Elevated body mass index is associated with increased integration and reduced cohesion of sensory-driven and internally guided resting-state functional brain networks. Cereb. Cortex 28, 988–997 (2018).
Caunca, M. R. et al. Measures of obesity are associated with MRI markers of brain aging: The Northern Manhattan Study. Neurology 93, 791–803 (2019).
Dake, M. D. et al. Obesity and brain vulnerability in normal and abnormal aging: a multimodal MRI study. J. Alzheimers Dis. Rep. 5, 65–77 (2021).
van Galen, K. A. et al. Brain responses to nutrients are severely impaired and not reversed by weight loss in humans with obesity: a randomized crossover study. Nat. Metab. 5, 1059–1072 (2023).
Dye, L., Boyle, N. B., Champ, C. & Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 76, 443–454 (2017).
Cournot, M. et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology 67, 1208–1214 (2006).
Yim, C. Y. et al. The effect of overweight/obesity on cognitive function in euthymic individuals with bipolar disorder. Eur. Psychiatry 27, 223–228 (2012).
Jaeger, J. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J. Clin. Psychopharmacol 38, 513–519 (2018).
Fama, R. & Sullivan, E. V. Thalamic structures and associated cognitive functions: relations with age and aging. Neurosci. Biobehav. Rev. 54, 29–37 (2015).
Burgaleta, M. et al. Subcortical regional morphology correlates with fluid and spatial intelligence. Hum. Brain Mapp. 35, 1957–1968 (2014).
Thibaut, F. Basal ganglia play a crucial role in decision making. Dialogues Clin. Neurosci. 18, 3 (2016).
Haber, S. N. & Knutson, B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35, 4–26 (2010).
Calsolaro, V. & Edison, P. Novel GLP-1 (glucagon-like peptide-1) analogues and insulin in the treatment for Alzheimer’s disease and other neurodegenerative diseases. CNS Drugs 29, 1023–1039 (2015).
Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011).
Aminoff, E. M., Kveraga, K. & Bar, M. The role of the parahippocampal cortex in cognition. Trends. Cogn. Sci. 17, 379–390 (2013).
Namboodiri, V. M. K. et al. Single-cell activity tracking reveals that orbitofrontal neurons acquire and maintain a long-term memory to guide behavioral adaptation. Nat. Neurosci. 22, 1110–1121 (2019).
Fry, A. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 186, 1026–1034 (2017).
Bosello, O. & Vanzo, A. Obesity paradox and aging. Eat. Weight Disord. 26, 27–35 (2021).
Heymsfield, S. B., Gallagher, D., Mayer, L., Beetsch, J. & Pietrobelli, A. Scaling of human body composition to stature: new insights into body mass index. Am. J. Clin. Nutr. 86, 82–91 (2007).
Yang, J. et al. Trajectories of depressive symptoms during pregnancy and risk of premature birth: a multicenter and prospective cohort study. Psychiatry Res. 326, 115284 (2023).
Zheng, M. et al. Nighttime sleep duration trajectories were associated with body mass index trajectories in early childhood. Pediatr. Obes. 16, e12766 (2020).
Reuter, M., Schmansky, N. J., Rosas, H. D. & Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage 61, 1402–1418 (2012).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23, S208–S219 (2004).
Greve, D. N. & Fischl, B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage 48, 63–72 (2009).
Finn, E. S. et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 18, 1664–1671 (2015).
Achard, S. & Bullmore, E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 3, e17 (2007).
He, Y., Chen, Z. & Evans, A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s disease. J. Neurosci. 28, 4756–4766 (2008).
Wang, J. et al. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum. Brain Mapp. 30, 1511–1523 (2009).
Bielczyk, N. Z. et al. Thresholding functional connectomes by means of mixture modeling. NeuroImage 171, 402–414 (2018).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage 52, 1059–1069 (2010).
Fawns-Ritchie, C. & Deary, I. J. Reliability and validity of the UK Biobank cognitive tests. PLoS ONE 15, e0231627 (2020).
Fanelli, G. et al. The link between cognition and somatic conditions related to insulin resistance in the UK Biobank study cohort: a systematic review. Neurosci. Biobehav. Rev. 143, 104927 (2022).
Arbuthnott, K. & Frank, J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J. Clin. Exp. Neuropsychol. 22, 518–528 (2000).
Tingley, D., Yamamoto, T., Hirose, K., Keele, L. & Imai, K. mediation: R package for causal mediation analysis. J. Stat. Softw. 59, 1–38 (2014).
Acknowledgements
This research is supported by the STI 2030 – Major Project (project no. 2022ZD0209000), the National Research Foundation, Singapore, and the Agency for Science Technology and Research (A*STAR), Singapore, under its Prenatal/Early Childhood Grant (grant no. H22P0M0007). Additional support is provided by the RGC GRF project (project no. 15201124) and the Hong Kong Global STEM Scholar scheme. We also thank S. Lyu (Department of Biomedical Engineering, National University of Singapore, Singapore) for his initial work on this study.
Author information
Authors and Affiliations
Contributions
A.Q. led the project. A.Q., D.Z, C.S., C.L. and N.C. were responsible for the study concept and the design of the study. D.Z., C.L. and C.S. contributed to brain image analysis and statistical analysis. C.S., J.H., K.K.L. and Z.W. contributed to visualization. A.Q., D.Z., J.H., K.K.L. and Z.W. contributed to the results interpretation and discussion. All the authors contributed to the manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Wyllians Borelli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information
Supplementary Information
Supplementary methods, Figures 1–4 and Tables 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, D., Shen, C., Chen, N. et al. Long-term obesity impacts brain morphology, functional connectivity and cognition in adults. Nat. Mental Health 3, 466–478 (2025). https://doi.org/10.1038/s44220-025-00396-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44220-025-00396-5
This article is cited by
-
Dynapenic abdominal obesity is associated with long-term cognitive trajectories and mild cognitive impairment
International Journal of Obesity (2026)
-
Regional adiposity shapes brain and cognition in adults
Nature Mental Health (2025)