Abstract
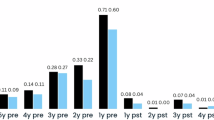
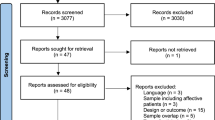
Switching to long-acting injectable (LAI) antipsychotic therapy as compared with continuation of oral therapy after a first episode of psychosis (FEP) may reduce the risk of relapse and hospitalization, as reported in some randomized trials. However, other trials and network meta-analyses reported no risk reduction. We emulated two target trials using data from the FEP-CAUSAL Collaboration, an international consortium of observational cohorts of people with FEP. The first target trial was designed to ask a similar question as the European Long-Acting Antipsychotics in Schizophrenia Trial (EULAST) trial, which compared the 18-month hospitalization risk between patients assigned to LAI therapy (aripiprazole, risperidone or paliperidone) and those on continuation of oral therapy. We benchmarked the observational estimates to those from the actual trial. The second target trial extended the first to examine the 3-year risks of psychotic relapses and in subgroups (prior relapses, non-adherence, substance use disorder). Of 2,228 individuals with FEP, 1,067 were eligible for the benchmarking analyses. Both our target trial emulation and EULAST showed little effect of LAI therapy initiation on the 18-month hospitalization risk. In the extended analysis (1,193 individuals), the 3-year risk difference of psychotic relapse comparing LAI therapy initiation with oral continuation was –7.0% (95% CI: –12.1, –0.7). The risk difference was substantially lower in subgroups with a prior relapse (–15.5%, 95%CI: –24.1, –5.5) or prior non-adherence (–21.9, 95% CI: –41.9, –2.0). We estimated that, compared with oral therapy continuation, LAI therapy initiation reduced psychotic relapses over 3 years. LAI therapy initiation may be particularly beneficial in vulnerable subgroups.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data are made available to the Coordinating Center of the FEP-CAUSAL Collaboration via data user agreements with each of the participating sites. Investigators interested in obtaining the data would need to establish similar arrangements.
Code availability
Code is available via GitHub at https://github.com/CausalInference/CAUSALab_Papers.
References
Arango, C. et al. Delphi panel to obtain clinical consensus about using long-acting injectable antipsychotics to treat first-episode and early-phase schizophrenia: treatment goals and approaches to functional recovery. BMC Psychiatry 23, 453 (2023).
Rauch, A. S. & Fleischhacker, W. W. Long-acting injectable formulations of new-generation antipsychotics: a review from a clinical perspective. CNS Drugs 27, 637–652 (2013).
Tiihonen, J. et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry 74, 686–693 (2017).
Solmi, M. et al. Effectiveness of antipsychotic use for reducing risk of work disability: results from a within-subject analysis of a Swedish national cohort of 21,551 patients with first-episode nonaffective psychosis. Am. J. Psychiatry 179, 938–946 (2022).
Boyer, L. et al. Real-world effectiveness of long-acting injectable antipsychotic treatments in a nationwide cohort of 12,373 patients with schizophrenia-spectrum disorders. Mol. Psychiatry 28, 3709–3716 (2023).
Tiihonen, J. et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. Br. Med. J. 333, 224 (2006).
Tiihonen, J. et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am. J. Psychiatry 168, 603–609 (2011).
Sancho-Echeverria, R. et al. Effect of long-acting antipsychotic treatment on psychiatric hospitalization rate in early psychosis patients: a naturalistic study. Ther. Adv. Psychopharmacol. 14, 20451253241243273 (2024).
Llorca, P. M. et al. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry 13, 340 (2013).
Brissos, S., Veguilla, M. R., Taylor, D. & Balanzá-Martinez, V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther. Adv. Psychopharmacol. 4, 198–219 (2014).
Hernán, M. A. How to estimate the effect of treatment duration on survival outcomes using observational data. Br. Med. J. 360, k182 (2018).
Szmulewicz, A. G. Target trial emulation in psychiatry: a call for more rigorous observational analyses. Lancet Psychiatry 10.1016/S2215-0366(24)00104-4 (2024).
Shahn, Z., Hernán, M. A. & Robins, J. M. A formal causal interpretation of the case-crossover design. Biometrics 79, 1330–1343 (2023).
Subotnik, K. L. et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry 72, 822–829 (2015).
Weiden, P. J. et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J. Clin. Psychiatry 70, 1397–1406 (2009).
Weiden, P. J., Schooler, N. R., Weedon, J. C., Elmouchtari, A. & Sunakawa-McMillan, A. Maintenance treatment with long-acting injectable risperidone in first-episode schizophrenia: a randomized effectiveness study. J. Clin. Psychiatry 73, 1224–1233 (2012).
Malla, A. et al. An exploratory, open-label, randomized trial comparing risperidone long-acting injectable with oral antipsychotic medication in the treatment of early psychosis. Clin. Schizophr. Relat. Psychoses 9, 198–208 (2016).
Kane, J. M. et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry 77, 1217–1224 (2020).
Efthimiou, O. et al. Efficacy and effectiveness of antipsychotics in schizophrenia: network meta-analyses combining evidence from randomised controlled trials and real-world data. Lancet Psychiatry 11, 102–111 (2024).
Schneider-Thoma, J. et al. Comparative efficacy and tolerability of 32 oral and long-acting injectable antipsychotics for the maintenance treatment of adults with schizophrenia: a systematic review and network meta-analysis. Lancet 399, 824–836 (2022).
Kishimoto, T., Hagi, K., Kurokawa, S., Kane, J. M. & Correll, C. U. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry 8, 387–404 (2021).
Ostuzzi, G., Bighelli, I., So, R., Furukawa, T. A. & Barbui, C. Does formulation matter? A systematic review and meta-analysis of oral versus long-acting antipsychotic studies. Schizophr. Res. 183, 10–21 (2017).
Winter-van Rossum, I. et al. Efficacy of oral versus long-acting antipsychotic treatment in patients with early-phase schizophrenia in Europe and Israel: a large-scale, open-label, randomised trial (EULAST). Lancet Psychiatry 10, 197–208 (2023).
Rivelli, A. et al. Real-world predictors of relapse in patients with schizophrenia and schizoaffective disorder in a large health system. Schizophrenia 10, 28 (2024).
Hui, C. L. M. et al. A 3-year retrospective cohort study of predictors of relapse in first-episode psychosis in Hong Kong. Aust. N. Z. J. Psychiatry 47, 746–753 (2013).
Szmulewicz, A. G. et al. Antipsychotic drugs in first-episode psychosis: a target trial emulation in the FEP-CAUSAL Collaboration. Am. J. Epidemiol. 193, 1081–1087 (2024).
Taipale, H. et al. Representation and outcomes of individuals with schizophrenia seen in everyday practice who are ineligible for randomized clinical trials. JAMA Psychiatry 79, 210–218 (2022).
Hernán, M. A. & Robins, J. M. Per-protocol analyses of pragmatic trials. N. Engl. J. Med. 377, 1391–1398 (2017).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th edn) (American Psychiatric Association, 1994).
Nesvåg, R., Hendset, M., Refsum, H. & Tanum, L. Serum concentrations of risperidone and 9-OH risperidone following intramuscular injection of long-acting risperidone compared with oral risperidone medication. Acta Psychiatr. Scand. 114, 21–26 (2006).
Rainer, M. K. Risperidone long-acting injection: a review of its long term safety and efficacy. Neuropsychiatr. Dis. Treat. 4, 919–927 (2008).
Pandina, G. et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry https://doi.org/10.1016/j.pnpbp.2010.11.008 (2011).
García-Albéniz, X., Hsu, J. & Hernán, M. A. The value of explicitly emulating a target trial when using real world evidence: an application to colorectal cancer screening. Eur. J. Epidemiol. 32, 495–500 (2017).
Acknowledgments
This study was funded by NIMH grant P50 MH115846-06 (D.Ö. and M.A.H.) and a BBRF Young Investigator Award (A.G.S). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
A.G.S. and G.M.-A. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. A.G.S., G.M.-A., D.Ö. and M.A.H. were involved in concept and study design. Acquisition and harmonization of datasets was conducted by G.M.-A., R.L., R.J., M.F., C.K., D.F., L.E.T., S.C., CM.D.-C., V.S., L.N.Y., D.K.S., A.K.S., C.A. and J.L.S. Analysis and interpretation of data was performed by A.G.S., G.M.-A., R.L., D.Ö. and M.A.H. The drafting of the manuscript was done by A.G.S. Critical revision of the manuscript for important intellectual content involved: G.M.-A., R.L., R.J., M.F., C.K., D.F., L.E.T., S.C., C.M.D.-C., V.S., L.N.Y., D.K.S., A.K.S., C.A. and J.L.S. The statistical analysis was performed by A.G.S. and M.A.H.; and the overall supervision by M.A.H. and D.Ö.
Corresponding author
Ethics declarations
Competing interests
D.O. served on the advisory board for Rapport Therapeutics in the past 12 months. C.M.D.-C. has received honoraria from Angelini and Viatris and travel support from Janssen and Angelini. M.A.H. Serves on the advisory board of ADIA Lab. C.A. has been a consultant to or has received honoraria or grants from Abbot, Acadia, Ambrosetti, Angelini, Biogen, BMS, Boehringer, Carnot, Gedeon Richter, Janssen Cilag, Lundbeck, Medscape, Menarini, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion, Takeda and Teva. L.N.Y. reports consultant/speaker fees from Alkermes, Allergan (currently Abbvie), Intracellular Therapies, LivaNova, Merck, Newron and Sumitomo Pharma and grants from Allergan (now AbbVie), CIHR and Sumitomo Pharma, outside the submitted work, over the past 3 years. He also received grants from Janssen, BMS, Otsuka, Lundbeck, Astra Zeneca and HLS. R.J. served as speaker and member of advisory board committees for Pfizer, Janssen, BMS, Sunovian, Myelin, Otsuka, Lundbeck, shire and Perdue. The other authors declare no competing interests.
Ethics statement
All research complies with the Declaration of Helsinki. The study was approved by the Harvard TH Chan School of Public Health Institutional Review Board (reference number IRB20-1842).
Peer review
Peer review information
Nature Mental Health thanks Hiroyoshi Takeuchi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, Tables 1–8 and Appendices 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Szmulewicz, A.G., Martínez-Alés, G., Logan, R. et al. Comparative effectiveness of long-acting injectable versus oral antipsychotic medication after a first episode of psychosis. Nat. Mental Health 3, 421–428 (2025). https://doi.org/10.1038/s44220-025-00407-5
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44220-025-00407-5