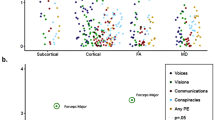
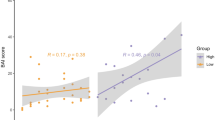
Abstract
Psychotic-like experiences (PLEs) may result from genetic and environmental risk factors that contribute to progressive declines in cognition and brain morphometry, which in turn exacerbate PLEs over time. Here we used three waves of unique longitudinal Adolescent Brain Cognitive Development Study data (ages 9–13 years) to test whether changes in cognition and global morphometry metrics attenuate associations between genetic and environmental risk with persistent distressing PLEs. Multigroup univariate latent growth models examined three waves of cognitive metrics and global morphometry separately for three PLEs groups: persistent distressing PLEs (n = 356), transient distressing PLEs (n = 408) and low-level PLEs (n = 7,901). Persistent distressing PLEs showed greater decreases (that is, more negative slopes) of cognition and morphometry metrics over time compared with those in low-level PLEs groups. Analyses also provided evidence for extant theories that worsening cognition and global morphometry metrics may partially account for associations between environmental risk with persistent distressing PLEs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data used in the preparation of this Article were obtained from the Adolescent Brain Cognitive Development (ABCD) study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 years and follow them over 10 years into early adulthood. The ABCD data repository grows and changes over time. The ABCD data used in this Article are available at https://nda.nih.gov/study.html?id=2313.
Code availability
Code is available via GitHub at https://github.com/nkarcher/Genetic-and-Environmental-Influences-on-Associations-between-Cognition-and-Neural-Metrics-with-PLEs.
References
Karcher, N. R. Psychotic-like experiences in childhood and early adolescence: clarifying the construct and future directions. Schizophr. Res. 246, 205–206 (2022).
Staines, L. et al. Psychotic experiences in the general population, a review; definition, risk factors, outcomes and interventions. Psychol. Med. 52, 3297–3308 (2022).
Fisher, H. L. et al. Specificity of childhood psychotic symptoms for predicting schizophrenia by 38 years of age: a birth cohort study. Psychol. Med. 43, 2077–2086 (2013).
Poulton, R. et al. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch. Gen. Psychiatry 57, 1053–1058 (2000).
Zammit, S. et al. Psychotic experiences and psychotic disorders at age 18 in relation to psychotic experiences at age 12 in a longitudinal population-based cohort study. Am. J. Psychiatry 170, 742–750 (2013).
Evermann, U., Gaser, C., Besteher, B., Langbein, K. & Nenadić, I. Cortical gyrification, psychotic-like experiences, and cognitive performance in nonclinical subjects. Schizophr. Bull. 46, 1524–1534 (2020).
Satterthwaite, T. D. et al. Structural brain abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry 73, 515–524 (2016).
Karcher, N. R. et al. Assessment of the Prodromal Questionnaire-Brief Child Version for Measurement of Self-reported Psychoticlike Experiences in Childhood. JAMA Psychiatry 75, 853–861 (2018).
Karcher, N. R. et al. Persistent and distressing psychotic-like experiences using Adolescent Brain Cognitive DevelopmentSM study data. Mol. Psychiatry 27, 1490–1501 (2022).
Schoorl, J. et al. Grey and white matter associations of psychotic-like experiences in a general population sample (UK Biobank). Transl. Psychiatry 11, 21 (2021).
Romer, A. L. & Pizzagalli, D. A. Associations between brain structural alterations, executive dysfunction, and general psychopathology in a healthy and cross-diagnostic adult patient sample. Biol. Psychiatry Glob. Open Sci. 2, 17–27 (2021).
Karcher, N. R., Merchant, J., Pine, J. & Kilciksiz, C. M. Cognitive dysfunction as a risk factor for psychosis. Curr. Top. Behav. Neurosci. 63, 173–203 (2023).
Gur, R. C. et al. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry 71, 366–374 (2014).
Dickson, H. et al. Trajectories of cognitive development during adolescence among youth at-risk for schizophrenia. J. Child Psychol. Psychiatry 59, 1215–1224 (2018).
Tamnes, C. K. et al. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J. Neurosci. 37, 3402–3412 (2017).
Thorup, A. A. E. et al. The Danish High-Risk and Resilience Study—VIA 15—a study protocol for the third clinical assessment of a cohort of 522 children born to parents diagnosed with schizophrenia or bipolar disorder and population-based controls. Front. Psychiatry 13, 809807 (2022).
Collins, M. A. et al. Accelerated cortical thinning precedes and predicts conversion to psychosis: the NAPLS3 longitudinal study of youth at clinical high-risk. Mol. Psychiatry 28, 1182–1189 (2023).
Cannon, T. D. et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol. Psychiatry 77, 147–157 (2015).
Pantelis, C. et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 361, 281–288 (2003).
Merritt, K., Luque Laguna, P., Irfan, A. & David, A. S. Longitudinal structural MRI findings in individuals at genetic and clinical high risk for psychosis: a systematic review. Front. Psychiatry 12, 620401 (2021).
van Os, J., Linscott, R. J., Myin-Germeys, I., Delespaul, P. & Krabbendam, L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol. Med. 39, 179–195 (2009).
Karcher, N. R. et al. Psychotic-like experiences and polygenic liability in the Adolescent Brain Cognitive Development Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 7, 45–55 (2022).
Karcher, N. R., Schiffman, J. & Barch, D. M. Environmental risk factors and psychotic-like experiences in children aged 9–10. J. Am. Acad. Child Adolesc. Psychiatry 60, 490–500 (2021).
Glenthøj, L. B., Hjorthøj, C., Kristensen, T. D., Davidson, C. A. & Nordentoft, M. The effect of cognitive remediation in individuals at ultra-high risk for psychosis: a systematic review. NPJ Schizophr. 3, 20 (2017).
Sekar, A. et al. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183 (2016).
Shaw, P. et al. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 28, 3586–3594 (2008).
Fusar-Poli, P. et al. Deconstructing vulnerability for psychosis: meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur. Psychiatry 40, 65–75 (2017).
Linscott, R. J. & van Os, J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol. Med. 43, 1133–1149 (2013).
Masten, A. S. & Cicchetti, D. Developmental cascades. Dev. Psychopathol. 22, 491–495 (2010).
Myin-Germeys, I. & van Os, J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin. Psychol. Rev. 27, 409–424 (2007).
Meyer, M. N. et al. Wrestling with social and behavioral genomics: risks, potential benefits, and ethical responsibility. Hastings Cent. Rep. 53, S2–S49 (2023).
Martin, A. R. et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 51, 584–591 (2019).
Karcher, N. R. & Barch, D. M. The ABCD study: understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology 46, 131–142 (2020).
Barch, D. M. et al. Demographic and mental health assessments in the adolescent brain and cognitive development study: updates and age-related trajectories. Dev. Cogn. Neurosci. 52, 101031 (2021).
Garavan, H. et al. Recruiting the ABCD sample: design considerations and procedures. Dev. Cogn. Neurosci. 32, 16–22 (2018).
Karcher, N. R., Modi, H., Kochunov, P., Gao, S. & Barch, D. M. Regional vulnerability indices in youth with persistent and distressing psychoticlike experiences. JAMA Netw. Open 6, e2343081 (2023).
Chapman, L. J., Chapman, J. P., Kwapil, T. R., Eckblad, M. & Zinser, M. C. Putatively psychosis-prone subjects 10 years later. J. Abnorm. Psychol. 103, 171–183 (1994).
Weintraub, S. et al. Cognition assessment using the NIH Toolbox. Neurology 80, S54–S64 (2013).
Fischl, B., Sereno, M. I. & Dale, A. M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage 9, 195–207 (1999).
Chen, C. H. et al. Hierarchical genetic organization of human cortical surface area. Science 335, 1634–1636 (2012).
Fischl, B. & Dale, A. M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl Acad. Sci. USA 97, 11050–11055 (2000).
Hagler, D. J. Jr et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage 202, 116091 (2019).
Beer, J. C. et al. Longitudinal ComBat: a method for harmonizing longitudinal multi-scanner imaging data. NeuroImage 220, 117129 (2020).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022).
Bigdeli, T. B. et al. Contributions of common genetic variants to risk of schizophrenia among individuals of African and Latino ancestry. Mol. Psychiatry 25, 2455–2467 (2020).
Ge, T., Chen, C. Y., Ni, Y., Feng, Y. C. A. & Smoller, J. W. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat. Commun. 10, 1776 (2019).
Rosenberg, J. M., Beymer, P. N., Anderson, D. J., van Lissa, C. J. & Schmidt, J. A. tidyLPA: an R package to easily carry out latent profile analysis (LPA) using open-source or commercial software. J. Open Source Softw. 3, 978 (2019).
Scrucca, L., Fop, M., Murphy, T. B. & Raftery, A. E. mclust 5: clustering, classification and density estimation using Gaussian finite mixture models. R. J. 8, 289–317 (2016).
Chakrabarti, A. & Ghosh, J. K. AIC, BIC and recent advances in model selection. Philos. Stat. 7, 583–605 (2011).
Acknowledgements
Data used in the preparation of this Article were obtained from the Adolescent Brain Cognitive Development (ABCD) study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 years and follow them over 10 years into early adulthood. The ABCD study is supported by the National Institutes of Health (NIH) and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123 and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This Article reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. This Article is the result of funding in whole or in part by the NIH. It is subject to the NIH Public Access Policy. Through acceptance of this federal funding, NIH has been given a right to make this Article publicly available in PubMed Central upon the Official Date of Publication, as defined by NIH. This work was supported by National Institutes of Health grants U01 DA041120 (D.M.B.), K23 MH121792 (N.R.K.), R01-MH139880 (N.R.K.), R01-DA054869 (A.A.), K01-DA051759 (E.C.J.), R01-DA054750 (A.A. and R.B.) and F31-AA029934 (S.E.P.).
Author information
Authors and Affiliations
Contributions
N.R.K. performed statistical analyses and prepared figures and tables. N.R.K., C.M.K. and D.M.B. drafted the paper. N.R.K., F.D. and D.M.B. designed the study. S.E.P. and E.C.J. computed PGS and performed quality assurance checks of genetic data. D.M.B. obtained funding. All authors, including N.R.K., F.D., S.E.P., E.C.J., C.M.K., H.O., J.S., A.A., R.B., J.J.J. and D.M.B., revised the paper and provided critical intellectual contributions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Pedro Mario Pan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Results, Tables 1–12 and Figs. 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karcher, N.R., Dong, F., Paul, S.E. et al. Cognitive and global morphometry trajectories as predictors of persistent distressing psychotic-like experiences in youth. Nat. Mental Health 3, 1012–1019 (2025). https://doi.org/10.1038/s44220-025-00481-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44220-025-00481-9