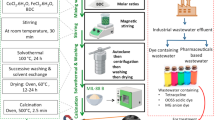
Abstract
Although Fenton and Fenton-like technologies have long been of great interest for application to environmental remediation, the transformation and final form of pollutants during the reaction have rarely been studied in depth. Here we report a pollutant transformation process, termed organic carbon transfer process (OCTP), in a Fenton-like reaction. Compared with the Fenton reaction previously reported for treating organic wastewater, the OCTP is very different and widely observed in reaction systems. In the OCTP, as oxidation proceeds and pollutant derivatives interact, the pollutants’ polarity changes and the pollutants predominantly accumulate on the catalyst surface. The OCTP occurs during the degradation of various wastewater types and accounts for up to 90.1% of the total substances accumulated on catalyst surfaces, even during industrial wastewater treatment. The in-depth study of OCTP has to some extent revealed the main reasons for the deactivation of heterogeneous catalysts during the reaction process and provided new research directions for the future study of heterogeneous catalytic systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the conclusions of this study are available within the manuscript and Supplementary information.
References
Tang, Z., Liu, Y., He, M. & Bu, W. Chemodynamic therapy: tumour microenvironment‐mediated Fenton and Fenton‐like reactions. Angew. Chem. Int. Ed. 58, 946–956 (2019).
Feng, Y. et al. Degradation of 14C-labeled few layer graphene via Fenton reaction: reaction rates, characterization of reaction products, and potential ecological effects. Water Res. 84, 49–57 (2015).
Yang, Z., Shan, C., Pignatello, J. J. & Pan, B. Mn(II) acceleration of the picolinic acid-assisted Fenton reaction: new insight into the role of manganese in homogeneous Fenton AOPs. Environ. Sci. Technol. 56, 6621–6630 (2022).
Luan, X. et al. Degradation of structurally defined graphene nanoribbons by myeloperoxidase and the photo‐Fenton reaction. Angew. Chem. Int. Ed. 59, 18515–18521 (2020).
Zhang, S., Zheng, H. & Tratnyek, P. G. Advanced redox processes for sustainable water treatment. Nat. Water 1, 666–681 (2023).
Wang, B. et al. A site distance effect induced by reactant molecule matchup in single‐atom catalysts for Fenton‐like reactions. Angew Chem. Int. Ed. 134, e202207268 (2022).
Yang, L. et al. Fe single‐atom catalyst for efficient and rapid Fenton‐like degradation of organics and disinfection against bacteria. Small 18, 2104941 (2022).
Tang, W. et al. Ru single atom catalyst with dual reaction sites for efficient Fenton-like degradation of organic contaminants. Appl. Catal. B 320, 121952 (2023).
Liu, L. et al. Nonradical activation of peroxydisulfate promoted by oxygen vacancy-laden NiO for catalytic phenol oxidative polymerization. Appl. Catal. B 254, 166–173 (2019).
Zhang, Y.-J. et al. Simultaneous nanocatalytic surface activation of pollutants and oxidants for highly efficient water decontamination. Nat. Commun. 13, 3005 (2022).
Cheng, C. et al. Generation of FeIV=O and its contribution to Fenton‐like reactions on a single‐atom iron−N−C catalyst. Angew Chem. Int. Ed. 62, e202218510 (2023).
Yin, K. et al. Microenvironment modulation of cobalt single-atom catalysts for boosting both radical oxidation and electron-transfer process in Fenton-like system. Appl. Catal. B 329, 122558 (2023).
Liang, X. et al. Coordination number dependent catalytic activity of single‐atom cobalt catalysts for Fenton‐like reaction. Adv. Funct. Mater. 32, 2203001 (2022).
Chen, F. et al. Single‐atom iron anchored tubular g‐C3N4 catalysts for ultrafast Fenton‐like reaction: roles of high‐valency iron‐oxo species and organic radicals. Adv. Mater. 34, 2202891 (2022).
Xiong, Y. et al. Single‐atom Fe catalysts for Fenton‐like reactions: roles of different N species. Adv. Mater. 34, 2110653 (2022).
Song, J. et al. Unsaturated single-atom CoN3 sites for improved Fenton-like reaction towards high-valent metal species. Appl. Catal. B 325, 122368 (2023).
Yang, M. et al. Versatile pathways for oxidating organics via peroxymonosulfate activation by different single atom catalysts confining with Fe–N4 or Cu–N4 sites. Chem. Eng. J. 451, 138606 (2023).
Zou, Y. et al. Tailoring the coordination environment of cobalt in a single-atom catalyst through phosphorus doping for enhanced activation of peroxymonosulfate and thus efficient degradation of sulfadiazine. Appl. Catal. B 312, 121408 (2022).
Li, Y., Zhang, W., Niu, J. & Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 6, 5164–5173 (2012).
Yan, Q., Zhang, J. & Xing, M. Cocatalytic Fenton reaction for pollutant control. Cell Rep. Phys. Sci. 1, 100149 (2020).
Pattanayak, S. et al. Spectroscopic and reactivity comparisons of a pair of bTAML complexes with FeV=O and FeIV=O units. Inorg. Chem. 56, 6352–6361 (2017).
Yan, Q. et al. Constructing an acidic microenvironment by MoS2 in heterogeneous Fenton reaction for pollutant control. Angew Chem. Int. Ed. 60, 17155–17163 (2021).
Lee, H. et al. Activation of persulfates by carbon nanotubes: oxidation of organic compounds by nonradical mechanism. Chem. Eng. J. 266, 28–33 (2015).
Ling, C. et al. Sulphur vacancy derived anaerobic hydroxyl radical generation at the pyrite-water interface: pollutants removal and pyrite self-oxidation behavior. Appl. Catal. B 290, 120051 (2021).
Li, X. et al. Enhanced atrazine degradation in the Fe(III)/peroxymonosulfate system via accelerating Fe(II) regeneration by benzoquinone. Chem. Eng. J. 427, 131995 (2022).
Zhao, Y. et al. α-Fe2O3 as a versatile and efficient oxygen atom transfer catalyst in combination with H2O as the oxygen source. Nat. Catal. 4, 684–691 (2021).
Chen, F. et al. Efficient decontamination of organic pollutants under high salinity conditions by a nonradical peroxymonosulfate activation system. Water Res. 191, 116799 (2021).
Jiang, J. et al. Photo-Fenton degradation of emerging pollutants over Fe-POM nanoparticle/porous and ultrathin g-C3N4 nanosheet with rich nitrogen defect: degradation mechanism, pathways, and products toxicity assessment. Appl. Catal. B 278, 119349 (2020).
Feng, C. & Loh, T.-P. Copper-catalyzed olefinic trifluoromethylation of enamides at room temperature. Chem. Sci. 3, 3458–3462 (2012).
Geng, W., Nakajima, T., Takanashi, H. & Ohki, A. Analysis of carboxyl group in coal and coal aromaticity by Fourier transform infrared (FT-IR) spectrometry. Fuel 88, 139–144 (2009).
Osmieri, L., Videla, A. H. M. & Specchia, S. Optimization of a Fe–N–C electrocatalyst supported on mesoporous carbon functionalized with polypyrrole for oxygen reduction reaction under both alkaline and acidic conditions. Int. J. Hydrogen Energy 41, 19610–19628 (2016).
Chen, X. et al. A novel double S-scheme photocatalyst Bi7O9I3/Cd0. 5Zn0. 5S QDs/WO3−x with efficient full-spectrum-induced phenol photodegradation. Appl. Catal. B 318, 121839 (2022).
Xing, M., Zhang, J., Chen, F. & Tian, B. An economic method to prepare vacuum activated photocatalysts with high photo-activities and photosensitivities. Chem. Commun. 47, 4947–4949 (2011).
Xing, M. Y., Qi, D. Y., Zhang, J. L. & Chen, F. One‐step hydrothermal method to prepare carbon and lanthanum co‐doped TiO2 nanocrystals with exposed {001} facets and their high UV and visible‐light photocatalytic activity. Chem. Eur. J. 41, 11432–11436 (2011).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (numbers 22325602 and 22176060) and Program of Shanghai Academic/Technology Research Leader (23XD1421000) and a project supported by Shanghai Municipal Science and Technology Major Project (grant number 2018SHZDZX03) and the Program of Introducing Talents of Discipline to Universities (B16017), as well as the Science and Technology Commission of Shanghai Municipality (20DZ2250400). Thanks to Q. Sui for helping us with PFCs detection. We thank the Research Center of Analysis and Test of East China University of Science and Technology for the help on the characterization.
Author information
Authors and Affiliations
Contributions
M.X. proposed the experimental concepts and supervised the project. M.X. and Z.C. designed the experiments and prepared the paper. Z.C., J.W., B.Y., J.L., Z.L., X.L. and Y.B. carried out the experiments and conducted the materials characterization. M.X., Z.C. and J.C. revised the paper. All authors have reviewed and agreed to submit the manuscript version and agree to be listed as co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Lizhi Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Texts 1–14, Figs. 1–53 and Tables 1–11.
Supplementary Data 1
Source data for supplementary figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Z., Wang, J., Yang, B. et al. Organic carbon transfer process in advanced oxidation systems for water clean-up. Nat Water 3, 334–344 (2025). https://doi.org/10.1038/s44221-025-00399-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44221-025-00399-7
This article is cited by
-
Neutral microenvironment-driven catalytic polymerization for closed-loop wastewater treatment and resource recovery
Nature Water (2026)
-
Universal scalable production of single-atom catalysts for antibiotic wastewater treatment
Nature Water (2026)
-
Breaking the oxo-wall for Co(IV)-oxo species and their nanoconfined catalytic performance within Ce-Co lamellar membrane
Nature Communications (2026)
-
From mineralization to low-carbon conversion: emerging paradigms in water decontamination
Science China Chemistry (2026)
-
Graphite Carbon Supported Single-Atom Iron Catalyst Activates Persulfate for Phenol Degradation: Radical and Non-Radical Synergistic Mechanism
Water, Air, & Soil Pollution (2026)