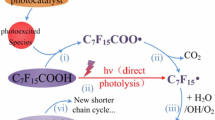
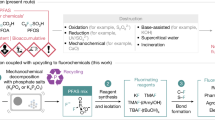
Abstract
Heterogeneous catalysis has the potential to efficiently and sustainably mineralize per- and polyfluoroalkyl substances (PFAS) with low material and energy inputs. However, the implementation of catalytic technologies is hindered by the large variety of PFAS compounds requiring treatment, a limited understanding of catalytic PFAS-degradation mechanisms and pathways, poor catalytic process selectivity towards PFAS over other water constituents, and a lack of appropriate methods to compare catalytic treatment options. Here we recommend strategies to overcome these challenges, including pretreating complex PFAS mixtures to simplify the design space of catalytic treatments, engineering catalytic systems and catalyst surfaces for selectivity, and developing holistic figures of merit that consider defluorination efficiencies and life-cycle costs to push forward the research, development and deployment of catalytic technologies for PFAS mineralization. Research needs to realize these designs and include a better understanding of the reaction mechanisms, catalyst surface engineering and treatment process design.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Uhlmann, D. M. PFAS Enforcement Discretion and Settlement Policy Under CERCLA (US EPA, 2024).
Per- and Polyfluoroalkyl Substances (PFAS) (US EPA, 2021); https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas
Wang, X. et al. Contamination of per- and polyfluoroalkyl substances in the water source from a typical agricultural area in North China. Front. Environ. Sci. 10.3389/fenvs.2022.1071134 (2023).
Directive (EU) 2020/2184 of the European Parliament and of the Council (European Council, 2020); https://eur-lex.europa.eu/eli/dir/2020/2184/oj
Boyer, T. H. et al. Life cycle environmental impacts of regeneration options for anion exchange resin remediation of PFAS impacted water. Water Res. 207, 117798 (2021).
Ellis, A. C., Boyer, T. H., Fang, Y., Liu, C. J. & Strathmann, T. J. Life cycle assessment and life cycle cost analysis of anion exchange and granular activated carbon systems for remediation of groundwater contaminated by per- and polyfluoroalkyl substances (PFASs). Water Res. 243, 120324 (2023).
Longendyke, G. K., Katel, S. & Wang, Y. PFAS fate and destruction mechanisms during thermal treatment: a comprehensive review. Environ. Sci. Process. Impacts 24, 196–208 (2022).
Wang, J. et al. Critical review of thermal decomposition of per- and polyfluoroalkyl substances: mechanisms and implications for thermal treatment processes. Environ. Sci. Technol. 56, 5355–5370 (2022).
Tow, E. W. et al. Managing and treating per- and polyfluoroalkyl substances (PFAS) in membrane concentrates. AWWA Water Sci. 3, e1233 (2021).
Fennell, B. D., Mezyk, S. P. & McKay, G. Critical review of UV-advanced reduction processes for the treatment of chemical contaminants in water. ACS Environ. Au 2, 178–205 (2022).
Cui, J., Gao, P. & Deng, Y. Destruction of per- and polyfluoroalkyl substances (PFAS) with advanced reduction processes (ARPs): a critical review. Environ. Sci. Technol. 54, 3752–3766 (2020).
Chen, Z. et al. Challenging the contamination of per- and polyfluoroalkyl substances in water: advanced oxidation or reduction? Environ. Funct. Mater. 1, 325–337 (2022).
Barzen-Hanson, K. A. et al. Discovery of 40 classes of per- and polyfluoroalkyl substances in historical aqueous film-forming foams (AFFFs) and AFFF-impacted groundwater. Environ. Sci. Technol. 51, 2047–2057 (2017).
Shojaei, M., Kumar, N. & Guelfo, J. L. An integrated approach for determination of total per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Technol. 56, 14517–14527 (2022).
Xiao, F., Jin, B., Golovko, S. A., Golovko, M. Y. & Xing, B. Sorption and desorption mechanisms of cationic and zwitterionic per- and polyfluoroalkyl substances in natural soils: thermodynamics and hysteresis. Environ. Sci. Technol. 53, 11818–11827 (2019).
Lenka, S. P., Kah, M. & Padhye, L. P. A review of the occurrence, transformation, and removal of poly- and perfluoroalkyl substances (PFAS) in wastewater treatment plants. Water Res. 199, 117187 (2021).
Thompson, K. A. et al. Poly- and perfluoroalkyl substances in municipal wastewater treatment plants in the United States: seasonal patterns and meta-analysis of long-term trends and average concentrations. ACS ES&T Water 2, 690–700 (2022).
D’Ambro, E. L. et al. Characterizing the air emissions, transport, and deposition of per- and polyfluoroalkyl substances from a fluoropolymer manufacturing facility. Environ. Sci. Technol. 55, 862–870 (2021).
Li, R. & MacDonald Gibson, J. Predicting the occurrence of short-chain PFAS in groundwater using machine-learned Bayesian networks. Front. Environ. Sci. 10.3389/fenvs.2022.958784 (2022).
Shimizu, M. S. et al. Atmospheric deposition and annual flux of legacy perfluoroalkyl substances and replacement perfluoroalkyl ether carboxylic acids in Wilmington, NC, USA. Environ. Sci. Technol. Lett. 8, 366–372 (2021).
Sun, M. et al. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the Cape Fear river watershed of North Carolina. Environ. Sci. Technol. Lett. 3, 415–419 (2016).
Smith, S. J. et al. The need to include a fluorine mass balance in the development of effective technologies for PFAS destruction. Environ. Sci. Technol. 58, 2587–2590 (2024).
Anumol, T., Dagnino, S., Vandervort, D. R. & Snyder, S. A. Transformation of polyfluorinated compounds in natural waters by advanced oxidation processes. Chemosphere 144, 1780–1787 (2016).
Houtz, E. F. & Sedlak, D. L. Oxidative conversion as a means of detecting precursors to perfluoroalkyl acids in urban runoff. Environ. Sci. Technol. 46, 9342–9349 (2012).
Ersan, M. S., Wang, B., Wong, M. S. & Westerhoff, P. Advanced oxidation processes may transform unknown PFAS in groundwater into known products. Chemosphere 349, 140865 (2024).
Cook, E. K. et al. Biological and chemical transformation of the six-carbon polyfluoroalkyl substance N-dimethyl ammonio propyl perfluorohexane sulfonamide (AmPr-FHxSA). Environ. Sci. Technol. 56, 15478–15488 (2022).
Houtz, E. F., Higgins, C. P., Field, J. A. & Sedlak, D. L. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ. Sci. Technol. 47, 8187–8195 (2013).
LaFond, J. A., Hatzinger, P. B., Guelfo, J. L., Millerick, K. & Andrew Jackson, W. Bacterial transformation of per- and poly-fluoroalkyl substances: a review for the field of bioremediation. Environ. Sci. Adv. 2, 1019–1041 (2023).
Jin, B. et al. Aerobic biotransformation and defluorination of fluoroalkylether substances (ether PFAS): substrate specificity, pathways and applications. Environ. Sci. Technol. Lett. 10, 755–761 (2023).
Liu, J. & Mejia Avendaño, S. Microbial degradation of polyfluoroalkyl chemicals in the environment: a review. Environ. Int. 61, 98–114 (2013).
Banayan Esfahani, E., Asadi Zeidabadi, F. & Mohseni, M. Vacuum-UV radiation capable of catalyst-free decomposition of 6:2 FTSA: the transformation mechanism and impacts of the water matrix. ACS ES&T Water 3, 3614–3625 (2023).
Chen, Z. et al. Enhanced UV photoreductive destruction of perfluorooctanoic acid in the presence of alcohols: synergistic mechanism of hydroxyl radical quenching and solvent effect. Appl. Catal. B 316, 121652 (2022).
Plumlee, M. H., McNeill, K. & Reinhard, M. Indirect photolysis of perfluorochemicals: hydroxyl radical-initiated oxidation of N-ethyl perfluorooctane sulfonamido acetate (N-EtFOSAA) and other perfluoroalkanesulfonamides. Environ. Sci. Technol. 43, 3662–3668 (2009).
Xin, X., Kim, J., Ashley, D. C. & Huang, C.-H. Degradation and defluorination of per- and polyfluoroalkyl substances by direct photolysis at 222 nm. ACS ES&T Water 3, 2776–2785 (2023).
Guan, Y. et al. Near-complete destruction of PFAS in aqueous film-forming foam by integrated photo-electrochemical processes. Nat. Water 2, 443–452 (2024).
Bentel, M. J. et al. Degradation of perfluoroalkyl ether carboxylic acids with hydrated electrons: structure–reactivity relationships and environmental implications. Environ. Sci. Technol. 54, 2489–2499 (2020).
Fennell, B. D., Chavez, S. & McKay, G. Destruction of per- and polyfluoroalkyl substances in reverse osmosis concentrate using UV-advanced reduction processes. ACS ES&T Water 4, 4818–4827 (2024).
Liu, Z. et al. Oxidative transformation of nafion-related fluorinated ether sulfonates: comparison with legacy PFAS structures and opportunities of acidic persulfate digestion for PFAS precursor analysis. Environ. Sci. Technol. 58, 6415–6424 (2024).
Zhang, C., Hopkins, Z. R., McCord, J., Strynar, M. J. & Knappe, D. R. U. Fate of per- and polyfluoroalkyl ether acids in the total oxidizable precursor assay and implications for the analysis of impacted water. Environ. Sci. Technol. Lett. 6, 662–668 (2019).
Ateia, M., Chiang, D., Cashman, M. & Acheson, C. Total Oxidizable Precursor (TOP) assay—best practices, capabilities and limitations for PFAS site investigation and remediation. Environ. Sci. Technol. Lett. 10, 292–301 (2023).
Minakata, D., Li, K., Westerhoff, P. & Crittenden, J. Development of a group contribution method to predict aqueous phase hydroxyl radical (HO•) reaction rate constants. Environ. Sci. Technol. 43, 6220–6227 (2009).
Minakata, D., Kamath, D. & Maetzold, S. Mechanistic insight into the reactivity of chlorine-derived radicals in the aqueous-phase UV-chlorine advanced oxidation process: quantum mechanical calculations. Environ. Sci. Technol. 51, 6918–6926 (2017).
Minakata, D. & Crittenden, J. Linear free energy relationships between aqueous phase hydroxyl radical reaction rate constants and free energy of activation. Environ. Sci. Technol. 45, 3479–3486 (2011).
Lee, J., von Gunten, U. & Kim, J.-H. Persulfate-based advanced oxidation: critical assessment of opportunities and roadblocks. Environ. Sci. Technol. 54, 3064–3081 (2020).
Radjenovic, J., Duinslaeger, N., Avval, S. S. & Chaplin, B. P. Facing the challenge of poly- and perfluoroalkyl substances in water: is electrochemical oxidation the answer? Environ. Sci. Technol. 54, 14815–14829 (2020).
Zhang, C., Tang, T. & Knappe, D. R. U. Oxidation of per- and polyfluoroalkyl ether acids and other per- and polyfluoroalkyl substances by sulfate and hydroxyl radicals: kinetic insights from experiments and models. Environ. Sci. Technol. 57, 18970–18980 (2023).
Liu, Z. et al. Near-quantitative defluorination of perfluorinated and fluorotelomer carboxylates and sulfonates with integrated oxidation and reduction. Environ. Sci. Technol. 55, 7052–7062 (2021).
Qanbarzadeh, M. et al. An ultraviolet/boron nitride photocatalytic process efficiently degrades poly-/perfluoroalkyl substances in complex water matrices. Environ. Sci. Technol. Lett. 10, 705–710 (2023).
Bentel, M. J. et al. Defluorination of per- and polyfluoroalkyl substances (PFASs) with hydrated electrons: structural dependence and implications to PFAS remediation and management. Environ. Sci. Technol. 53, 3718–3728 (2019).
Fang, B. et al. Stability and biotransformation of 6:2 fluorotelomer sulfonic acid, sulfonamide amine oxide and sulfonamide alkylbetaine in aerobic aludge. Environ. Sci. Technol. 58, 2446–2457 (2024).
Le, T. X. H., Haflich, H., Shah, A. D. & Chaplin, B. P. Energy-efficient electrochemical oxidation of perfluoroalkyl substances using a Ti4O7 reactive electrochemical membrane anode. Environ. Sci. Technol. Lett. 6, 504–510 (2019).
Yin, S. et al. Trap-n-zap: electrocatalytic degradation of perfluorooctanoic acid (PFOA) with UiO-66 modified boron nitride electrodes at environmentally relevant concentrations. Appl. Catal. B Environ. Energy 355, 124136 (2024).
Chen, Y. et al. Mechanistic insight into the photo-oxidation of perfluorocarboxylic acid over boron nitride. Environ. Sci. Technol. 56, 8942–8952 (2022).
Long, M. et al. Adsorption and reductive defluorination of perfluorooctanoic acid over palladium nanoparticles. Environ. Sci. Technol. 55, 14836–14843 (2021).
Long, M., Zheng, C.-W., Roldan, M. A., Zhou, C. & Rittmann, B. E. Co-removal of perfluorooctanoic acid and nitrate from water by coupling Pd catalysis with enzymatic biotransformation. Environ. Sci. Technol. 58, 11514–11524 (2024).
Long, M. et al. Hydrodefluorination of perfluorooctanoic acid in the H2-based membrane catalyst-film reactor with platinum group metal nanoparticles: pathways and optimal conditions. Environ. Sci. Technol. 55, 16699–16707 (2021).
Wu, B. et al. Rapid destruction and defluorination of perfluorooctanesulfonate by alkaline hydrothermal reaction. Environ. Sci. Technol. Lett. 6, 630–636 (2019).
Hao, S., Choi, Y. J., Deeb, R. A., Strathmann, T. J. & Higgins, C. P. Application of hydrothermal alkaline treatment for destruction of per- and polyfluoroalkyl substances in contaminated groundwater and soil. Environ. Sci. Technol. 56, 6647–6657 (2022).
Yu, Y. et al. Microbial cleavage of C-F bonds in two C6 per- and polyfluorinated compounds via reductive defluorination. Environ. Sci. Technol. 54, 14393–14402 (2020).
Che, S. et al. Structure-specific aerobic defluorination of short-chain fluorinated carboxylic acids by activated sludge communities. Environ. Sci. Technol. Lett. 8, 668–674 (2021).
Zhang, Z. & Ma, Y. The path to complete defluorination of PFAS. Nat. Water 1, 313–314 (2023).
Biswas, S. & Wong, B. M. Beyond conventional density functional theory: advanced quantum dynamical methods for understanding degradation of per- and polyfluoroalkyl substances. ACS ES&T Eng. 4, 96–104 (2024).
Zhang, Y., Moores, A., Liu, J. & Ghoshal, S. New insights into the degradation mechanism of perfluorooctanoic acid by persulfate from density functional theory and experimental data. Environ. Sci. Technol. 53, 8672–8681 (2019).
Raza, A. et al. A machine learning approach for predicting defluorination of per- and polyfluoroalkyl substances (PFAS) for their efficient treatment and removal. Environ. Sci. Technol. Lett. 6, 624–629 (2019).
Awfa, D., Ateia, M., Mendoza, D. & Yoshimura, C. Application of quantitative structure-property relationship predictive models to water treatment: a critical review. ACS ES&T Water 1, 498–517 (2021).
Minakata, D. & von Gunten, U. Predicting transformation products during aqueous oxidation processes: current state and outlook. Environ. Sci. Technol. 57, 18410–18419 (2023).
Zhang, K., Zhong, S. & Zhang, H. Predicting aqueous adsorption of organic compounds onto biochars, carbon nanotubes, granular activated carbons and resins with machine learning. Environ. Sci. Technol. 54, 7008–7018 (2020).
Zhang, T. et al. In situ remediation of subsurface contamination: opportunities and challenges for nanotechnology and advanced materials. Environ. Sci. Nano 6, 1283–1302 (2019).
Arana Juve, J.-M. et al. Size-selective trapping and photocatalytic degradation of PFOA in Fe-modified zeolite frameworks. Appl. Catal. B Environ. Energy 349, 123885 (2024).
Van den Bergh, M. et al. Highly selective removal of perfluorinated contaminants by adsorption on all-silica zeolite beta. Angew. Chem. Int. Ed. 59, 14086–14090 (2020).
Chen, Z. et al. Amine-functionalized A-center sphalerite for selective and efficient destruction of perfluorooctanoic acid. Environ. Sci. Technol. 57, 10438–10447 (2023).
Fu, H. et al. Mechanism for selective binding of aromatic compounds on oxygen-rich graphene nanosheets based on molecule size/polarity matching. Sci. Adv. 8, eabn4650 (2022).
Bouteh, E., Bentel, M. J. & Cates, E. L. Semiconductor-hydrophobic material interfaces as a new active site paradigm for photocatalytic degradation of perfluorocarboxylic acids. J. Hazard. Mater. 453, 131437 (2023).
Lee, C.-G. et al. Porous electrospun fibers embedding TiO2 for adsorption and photocatalytic degradation of water pollutants. Environ. Sci. Technol. 52, 4285–4293 (2018).
Wang, B. et al. Surface hydrophobicity of boron nitride promotes PFOA photocatalytic degradation. Chem. Eng. J. 483, 149134 (2024).
Wang, Y. et al. Molecularly imprinted MOFs-driven carbon nanofiber for sensitive electrochemical detection and targeted electro-Fenton degradation of perfluorooctanoic acid. Sep. Purif. Technol. 310, 123257 (2023).
Li, F. et al. Biomimetic degradability of linear perfluorooctanesulfonate (L-PFOS): degradation products and pathways. Chemosphere 259, 127502 (2020).
Marciesky, M., Aga, D. S., Bradley, I. M., Aich, N. & Ng, C. Mechanisms and opportunities for rational in silico design of enzymes to degrade per- and polyfluoroalkyl substances (PFAS). J. Chem. Inf. Model. 63, 7299–7319 (2023).
Park, S., de Perre, C. & Lee, L. S. Alternate reductants with VB12 to transform C8 and C6 perfluoroalkyl sulfonates: limitations and insights into isomer-specific transformation rates, products and pathways. Environ. Sci. Technol. 51, 13869–13877 (2017).
Yu, Y. et al. Microbial defluorination of unsaturated per- and polyfluorinated carboxylic acids under anaerobic and aerobic conditions: a structure specificity study. Environ. Sci. Technol. 56, 4894–4904 (2022).
McDonough, C. A., Guelfo, J. L. & Higgins, C. P. Measuring total PFASs in water: the tradeoff between selectivity and inclusivity. Curr. Opin. Environ. Sci. Health 7, 13–18 (2019).
Liu, Y., D’Agostino, L. A., Qu, G., Jiang, G. & Martin, J. W. High-resolution mass spectrometry (HRMS) methods for nontarget discovery and characterization of poly- and per-fluoroalkyl substances (PFASs) in environmental and human samples. Trends Anal. Chem. 121, 115420 (2019).
Bowers, B. B. et al. Nontarget analysis and fluorine atom balances of transformation products from UV/sulfite degradation of perfluoroalkyl contaminants. Environ. Sci. Process. Impacts 25, 472–483 (2023).
Tenorio, R., Maizel, A. C., Schaefer, C. E., Higgins, C. P. & Strathmann, T. J. Application of high-resolution mass spectrometry to evaluate UV-sulfite-induced transformations of per- and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam (AFFF). Environ. Sci. Technol. 56, 14774–14787 (2022).
Lauria, M. Z. et al. Closing the organofluorine mass balance in marine mammals using suspect screening and machine learning-based quantification. Environ. Sci. Technol. 58, 2458–2467 (2024).
Charbonnet, J. A. et al. Environmental source tracking of per- and polyfluoroalkyl substances within a forensic context: current and future techniques. Environ. Sci. Technol. 55, 7237–7245 (2021).
Joseph, N. T. et al. Target and suspect screening integrated with machine learning to discover per- and polyfluoroalkyl substance source fingerprints. Environ. Sci. Technol. 57, 14351–14362 (2023).
Horst, J., McDonough, J., Ross, I. & Houtz, E. Understanding and managing the potential by-products of PFAS destruction. Groundwater Monit. Rem. 40, 17–27 (2020).
Bolton, J. R., Bircher, K. G., Tumas, W. & Tolman, C. A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 73, 627–637 (2001).
Miklos, D. B. et al. Evaluation of advanced oxidation processes for water and wastewater treatment—a critical review. Water Res. 139, 118–131 (2018).
Sun, Z., Zhang, C., Chen, P., Zhou, Q. & Hoffmann, M. R. Impact of humic acid on the photoreductive degradation of perfluorooctane sulfonate (PFOS) by UV/iodide process. Water Res. 127, 50–58 (2017).
Tushar, M. M. R., Pushan, Z. A., Aich, N. & Rowles, L. S. Balancing sustainability goals and treatment efficacy for PFAS removal from water. npj Clean Water 7, 130 (2024).
Glass, S. et al. Supporting information: Merits, limitations, and innovation priorities for heterogeneous catalytic platforms to destroy PFAS. Zenodo https://zenodo.org/records/14956697 (2025).
Liu, S. et al. Promotive effects of chloride and sulfate on the near-complete destruction of perfluorocarboxylates (PFCAs) in brine via hydrogen-tuned 185-nm UV photolysis: mechanisms and kinetics. Environ. Sci. Technol. 58, 10347–10356 (2024).
Liu, Z. et al. Accelerated degradation of perfluorosulfonates and perfluorocarboxylates by UV/sulfite + iodide: reaction mechanisms and system efficiencies. Environ. Sci. Technol. 56, 3699–3709 (2022).
Zarzo, D. & Prats, D. Desalination and energy consumption. What can we expect in the near future? Desalination 427, 1–9 (2018).
Liu, C. J. et al. Pilot-scale field demonstration of a hybrid nanofiltration and UV-sulfite treatment train for groundwater contaminated by per- and polyfluoroalkyl substances (PFASs). Water Res. 205, 117677 (2021).
Rosenfeldt, E. J., Linden, K. G., Canonica, S. & von Gunten, U. Comparison of the efficiency of OH radical formation during ozonation and the advanced oxidation processes O3/H2O2 and UV/H2O2. Water Res. 40, 3695–3704 (2006).
Qu, X., Alvarez, P. J. J. & Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 47, 3931–3946 (2013).
Appleman, T. D., Dickenson, E. R. V., Bellona, C. & Higgins, C. P. Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. J. Hazard. Mater. 260, 740–746 (2013).
Acknowledgements
This work was partially funded by the National Science Foundation Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment (EEC-1449500). G.V.L. and H.A.S.-C. acknowledge funding support from the NIEHS (R01ES032708) for their contributions. H.A.S.-C. acknowledges support from the National Science Foundation Graduate Research Fellowship (DGE2140739) and a Dean’s Fellowship from the College of Engineering at Carnegie Mellon University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. W.C. and T.Z. acknowledge support from the National Natural Science Foundation of China (22125603 and 22020102004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Timothy Strathmann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Glass, S., Santiago-Cruz, H.A., Chen, W. et al. Merits, limitations and innovation priorities for heterogeneous catalytic platforms to destroy PFAS. Nat Water 3, 644–654 (2025). https://doi.org/10.1038/s44221-025-00433-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44221-025-00433-8