Abstract
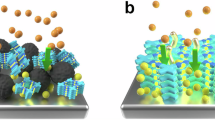
Environmental remediation of uranium-containing wastewater has emerged as a critical challenge in the nuclear industry, addressing both ecological protection and resource sustainability concerns. However, conventional adsorption methods suffer from slow kinetics, whereas electrochemical approaches are limited by the direct coupling between electron injection and uranyl ion reduction, leading to ion-blocking layers that severely impede remediation efficiency. Here we develop an electron-buffering rechargeable microelectrode adsorbent system that operates through a three-step cycle: electron storage, uranium extraction and adsorbent regeneration. This sequential process decouples electron injection from uranyl ion reduction by separating them in time and space. The microelectrode stores electrons in an environment free of competing ions and releases them in a controlled manner during uranium extraction. The Fe–O bonds on the surface serve as active sites for capturing uranyl ions and lowering their reduction overpotential, whereas the release of charge-balancing cations maintains surface electronegativity for efficient mass transfer. This synergistic integration achieves an initial extraction rate of 1,062 mg g−1 h−1 and a uranium capacity of 854 mg g−1, with nearly 100% electron utilization efficiency. Most importantly, when tested with actual uranium mine wastewater containing 0.545 ppm uranium, the system achieves 97.1% extraction efficiency and a capacity of 78.5 mg g−1 within 6 h, demonstrating its practical viability for environmental remediation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data that supports the findings of this study are available within the article and its Supplementary Information.
Change history
28 August 2025
In the version of Supplementary Information initially published alongside this article, references in Table 1 cited papers in the main article reference list, and now cite references in a revised Supplementary References list.
References
Uranium 2022: Resources, Production and Demand (NEA & IAEA, 2023).
Xie, Y. et al. Uranium extraction from seawater: material design, emerging technologies and marine engineering. Chem. Soc. Rev. 52, 97–162 (2023).
The Uranium Fuel Report: Expanded Summary (WNA, 2022).
Hu, Y. et al. Photochemically triggered self-extraction of uranium from aqueous solution under ambient conditions. Appl. Catal. B. 322, 122092 (2023).
Li, P. et al. Uranium elimination and recovery from wastewater with ligand chelation-enhanced electrocoagulation. Chem. Eng. J. 393, 124819 (2020).
Hua, Y. et al. Enrichment of uranium from wastewater with nanoscale zero-valent iron (nZVI). Environ. Sci. Nano 8, 666–674 (2021).
Fang, Q. et al. High-efficiency removal of U(VI) from low-concentration uranium-bearing wastewater using ZnCl2-modified activated carbon loading nZVI. J. Radioanal. Nucl. Chem. 332, 3977–3990 (2023).
Lin, T. et al. Ion pair sites for efficient electrochemical extraction of uranium in real nuclear wastewater. Nat. Commun. 15, 4149 (2024).
Li, J. et al. Removal of uranium from uranium plant wastewater using zero-valent iron in an ultrasonic field. Nucl. Eng. Technol. 48, 744–750 (2016).
Yang, P. et al. Water-endurable intercalated graphene oxide adsorbent with highly efficient uranium capture from acidic wastewater. Sep. Purif. Technol. 263, 118364 (2021).
Ye, Y. et al. Spontaneous electrochemical uranium extraction from wastewater with net electrical energy production. Nat. Water 1, 887–898 (2023).
Zhao, S. et al. A dual-surface amidoximated halloysite nanotube for high-efficiency economical uranium extraction from seawater. Angew. Chem. Int. Ed. 58, 14979–14985 (2019).
Tang, N. et al. Amidoxime-based materials for uranium recovery and removal. J. Mater. Chem. A 8, 7588–7625 (2020).
Yang, L. et al. Bioinspired hierarchical porous membrane for efficient uranium extraction from seawater. Nat. Sustain. 5, 71–80 (2021).
Liu, T. et al. Vertically aligned polyamidoxime/graphene oxide hybrid sheets’ membrane for ultrafast and selective extraction of uranium from seawater. Adv. Funct. Mater. 32, 2111049 (2022).
Li, H. et al. Zwitterion functionalized graphene oxide/polyacrylamide/polyacrylic acid hydrogels with photothermal conversion and antibacterial properties for highly efficient uranium extraction from seawater. Adv. Funct. Mater. 33, 2301773 (2023).
Zhang, C.-R. et al. rGO-based covalent organic framework hydrogel for synergistically enhance uranium capture capacity through photothermal desalination. Chem. Eng. J. 428, 131178 (2022).
Fu, M. et al. Solar enhanced uranium extraction from seawater with the efficient strategy of MXene loaded nano-porous polyamidoxime membrane. Sep. Purif. Technol. 332, 125803 (2024).
Sun, Q. et al. Bio-inspired nano-traps for uranium extraction from seawater and recovery from nuclear waste. Nat. Commun. 9, 1644 (2018).
Xu, X. et al. 3D hierarchical porous amidoxime fibers speed up uranium extraction from seawater. Energy Environ. Sci. 12, 1979–1988 (2019).
Yuan, Y. et al. Photoinduced multiple effects to enhance uranium extraction from natural seawater by black phosphorus nanosheets. Angew. Chem. Int. Ed. 59, 1220–1227 (2019).
Yan, B. et al. An ion-crosslinked supramolecular hydrogel for ultrahigh and fast uranium recovery from seawater. Adv. Mater. 32, 1906615 (2020).
Li, N. et al. High-capacity amidoxime-functionalized β-cyclodextrin/graphene aerogel for selective uranium capture. Environ. Sci. Technol. 55, 9181–9188 (2021).
Li, T. et al. Photothermal-enhanced uranium extraction from seawater: a biomass solar thermal collector with 3D ion-transport networks. Adv. Funct. Mater. 33, 2212819 (2023).
Liu, C. et al. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat. Energy 2, 17007 (2017).
Chi, F. et al. Highly efficient recovery of uranium from seawater using an electrochemical approach. Ind. Eng. Chem. Res. 57, 8078–8084 (2018).
Xue, Y. et al. Electroadsorption of uranium on amidoxime modified graphite felt. Sep. Purif. Technol. 255, 117753 (2021).
Ye, Y. et al. Electrochemical removal and recovery of uranium: effects of operation conditions, mechanisms, and implications. J. Hazard. Mater. 432, 128723 (2022).
Liu, X. et al. Highly efficient electrocatalytic uranium extraction from seawater over an amidoxime‐functionalized In–N–C catalyst. Adv. Sci. 9, 2201735 (2022).
Yang, H. et al. Functionalized iron–nitrogen–carbon electrocatalyst provides a reversible electron transfer platform for efficient uranium extraction from seawater. Adv. Mater. 33, 2106621 (2021).
Zhang, P. et al. Aryl diazonium-assisted amidoximation of MXene for boosting water stability and uranyl sequestration via electrochemical sorption. ACS Appl. Mater. Interfaces 12, 15579–15587 (2020).
Pan, M. et al. Efficient electrosorption of uranyl ions by a homemade amidoxime-modified carbon paper-based electrode in acidic aqueous condition. J. Chem. Technol. Biotechnol. 96, 2916–2929 (2021).
Tang, X. et al. Sulfur edge in molybdenum disulfide nanosheets achieves efficient uranium binding and electrocatalytic extraction in seawater. Nanoscale 14, 6285–6290 (2022).
Lin, L. et al. Electrocatalytic removal of low-concentration uranium using TiO2 nanotube arrays/Ti mesh electrodes. Environ. Sci. Technol. 56, 13327–13337 (2022).
Wang, Y. et al. Electrochemical-mediated regenerable FeII active sites for efficient uranium extraction at ultra-low cell voltage. Angew. Chem. Int. Ed. 62, e202217601 (2023).
Feng, S. et al. Stabilizing *CO2 intermediates at the acidic interface using molecularly dispersed cobalt phthalocyanine as catalysts for CO2 reduction. Angew. Chem. Int. Ed. 63, e202317942 (2024).
Shen, M. et al. Hierarchical design enables sufficient activated CO2 for efficient electrolysis of bicarbonate to CO. Joule 8, 1999–2015 (2024).
Chen, Y. et al. Preparation of porous composite phase Na super ionic conductor adsorbent by in situ process for ultrafast and efficient strontium adsorption from wastewater. Metals 13, 677 (2023).
Liu, N. et al. Fe-MMT/WO3 composites for chemical and photocatalysis synergistic reduction of uranium (VI). Chemosphere 344, 140321 (2023).
Chen, Y. et al. Flexible self-supporting Na3MnTi(PO4)3@C fibres for uranium extraction from seawater by electro sorption. J. Hazard. Mater. 461, 132664 (2024).
Yu, K. et al. Semiconducting metal–organic frameworks decorated with spatially separated dual cocatalysts for efficient uranium(VI) photoreduction. Adv. Funct. Mater. 32, 2200315 (2022).
Wang, C. et al. Dual-functional S-scheme Fe3O4/TiO2/g-C3N4 double-heterostructure bridged by TiO2 for collaborative removal of U(VI) and Sb(III). J. Cleaner Prod. 426, 139114 (2023).
Dong, Z. et al. Novel Co3O4@TiO2@CdS@Au double-shelled nanocage for high-efficient photocatalysis removal of U(VI): roles of spatial charges separation and photothermal effect. J. Hazard. Mater. 452, 131248 (2023).
Yan, G. et al. Defining the challenges of Li extraction with olivine host: the roles of competitor and spectator ions. Proc. Natl Acad. Sci. USA 119, e2200751119 (2022).
Zhang, T. et al. Constructing new Fe3O4@MnOx with 3D hollow structure for efficient recovery of uranium from simulated seawater. Chemosphere 283, 131241 (2021).
Li, N. et al. Bioinspired green tea waste/graphene aerogel for solar-enhanced uranium extraction from seawater. Desalination 545, 116153 (2023).
Liu, T. et al. Mussel-inspired dual-crosslinked polyamidoxime photothermal hydrogel with enhanced mechanical strength for highly efficient and selective uranium extraction from seawater. Chem. Eng. J. 430, 133182 (2022).
Ho, Y. et al. Pseudo-second order model for sorption processes. Process Biochem. 34, 451–465 (1999).
Zhang, H. et al. Three mechanisms in one material: uranium capture by a polyoxometalate-organic framework through combined complexation, chemical reduction, and photocatalytic reduction. Angew. Chem. Int. Ed. 58, 16110–16114 (2019).
Cui, W. R. et al. Regenerable covalent organic frameworks for photo-enhanced uranium adsorption from seawater. Angew. Chem. Int. Ed. 59, 17684–17690 (2020).
Zhang, X. et al. Controllable shell corrosion of coated nanoscale zero valent iron induces long-term potentiation of its reactivity for uranium removal. Sep. Purif. Technol. 287, 120550 (2022).
Latta, E. et al. Influence of magnetite stoichiometry on UVI reduction. Environ. Sci. Technol. 46, 778–786 (2012).
Ye, Y. et al. Efficient and durable uranium extraction from uranium mine tailings seepage water via a photoelectrochemical method. iScience 24, 103230 (2021).
AbowSlama, E. H. Y., Ebraheem, E. & Sam, A. K. Precipitation and purification of uranium from rock phosphate. J. Radioanal. Nucl. Chem. 299, 815–818 (2013).
Rychkov, V. N. et al. Precipitation of yellowcake from pregnant regenerate by various reagents. J. Radioanal. Nucl. Chem. 314, 1741–1746 (2017).
Smirnov, A. L. et al. Precipitation of uranium from nitrate-sulfuric eluates by aqueous ammonia solution. J. Radioanal. Nucl. Chem. 317, 863–869 (2018).
Acknowledgements
This work is jointly supported by the National Natural Science Foundation of China (numbers 92462301, 52525202, 92262305, 52302225, 52202251, 52461160296), ‘GeoX’ Interdisciplinary Project of Frontiers Science Center for Critical Earth Material Cycling (20250106), the New Cornerstone Science Foundation through XPLORER PRIZE, the Natural Science Foundation of Jiangsu Province (BK20233001, BK20243009) and the Project of Laoshan Laboratory (LSKJ2025001600). We acknowledge the micro-fabrication centre of the National Laboratory of Solid State Microstructures (NLSSM) for technique support.
Author information
Authors and Affiliations
Contributions
J.Z. and Xiaojun Wang conceived the project and designed the experiments. S.C., H.Z., Z.W., Xiyang Wang and Z.L. conducted the synthesis, characterizations and the majority of the experiments. S.C. and S.F. were responsible for device fabrication. C.Z. and T.S. provided critical resources and valuable guidance. S.C., Xiaojun Wang and J.Z. analysed the data and drafted the manuscript. All authors contributed to discussions of the results and provided feedback on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Shaohong Liu, Shengqian Ma and Costas Tsouris for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–32 and Tables 1–4.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Wang, X., Zheng, H. et al. Electron-buffering rechargeable microelectrode adsorbents for rapid environmental remediation of uranium-containing wastewater. Nat Water 3, 937–948 (2025). https://doi.org/10.1038/s44221-025-00471-2
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44221-025-00471-2
This article is cited by
-
Harvesting uranium from nuclear waste streams
Nature Water (2025)