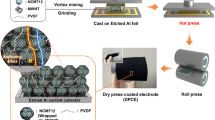
Abstract
Lithium-ion batteries (LIBs) need to be manufactured at speed and scale for their use in electric vehicles and devices. However, LIB electrode manufacturing via conventional wet slurry processing is energy-intensive and costly, challenging the goal to achieve sustainable, affordable and facile manufacturing of high-performance LIBs. In this Review, we discuss advanced electrode processing routes (dry processing, radiation curing processing, advanced wet processing and 3D-printing processing) that could reduce energy usage and material waste. Maxwell-type dry processing is a scalable alternative to conventional processing and has relatively low manufacturing cost and energy consumption. Radiation curing processing could enable high-throughput manufacturing, but binder selection is limited to certain radiation curable chemistries. 3D-printing processing can produce electrodes with diverse architectures and improved rate performance, but scalability is yet to be demonstrated. 3D-printing processing is good for special applications where throughput and cost can be compromised for performance.
Key points
-
Conventional lithium-ion battery electrode processing heavily relies on wet processing, which is time-consuming and energy-consuming.
-
Compared with conventional routes, advanced electrode processing strategies can be more affordable and less energy-intensive and generate less waste.
-
Electrode architectures can be tailored through advanced wet processing to improve charge and discharge rate performance, at the expense of increased manufacturing cost.
-
Dry processing can simplify the electrode manufacturing process with lower manufacturing costs (~11.5%) and energy consumption (>46% lower).
-
Radiation curing technologies can have the highest electrode manufacturing throughput, whereas 3D printing can fabricate electrodes with different geometries and structures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rajaeifar, M. A., Ghadimi, P., Raugei, M., Wu, Y. & Heidrich, O. Challenges and recent developments in supply and value chains of electric vehicle batteries: a sustainability perspective. Resour. Conserv. Recycl. 180, 106144 (2022).
Goodenough, J. B. How we made the Li-ion rechargeable battery. Nat. Electron. 1, 204 (2018).
Tao, R. et al. Insight into the fast-rechargeability of a novel Mo1.5W1.5Nb14O44 anode material for high-performance lithium-ion batteries. Adv. Energy Mater. 12, 2200519 (2022).
Tarascon, J. M. & Guyomard, D. The Li1+xMn2O4/C rocking-chair system: a review. Electrochim. Acta 38, 1221–1231 (1993).
Li, J., Fleetwood, J., Hawley, W. B. & Kays, W. From materials to cell: state-of-the-art and prospective technologies for lithium-ion battery electrode processing. Chem. Rev. 122, 903–956 (2022).
Zhang, Y. et al. Recent technology development in solvent-free electrode fabrication for lithium-ion batteries. Renew. Sustain. Energy Rev. 183, 113515 (2023).
Li, J., Daniel, C. & Wood III, D. L. in Handbook of Battery Materials (eds Daniel, C. & Besenhard, J. O.) 939–960 (Wiley, 2011).
Liu, Y., Zhang, R., Wang, J. & Wang, Y. Current and future lithium-ion battery manufacturing. iScience 24, 102332 (2021).
Wood, D. L. et al. Technical and economic analysis of solvent-based lithium-ion electrode drying with water and NMP. Dry. Technol. 36, 234–244 (2018).
Ahmed, S., Nelson, P. A., Gallagher, K. G. & Dees, D. W. Energy impact of cathode drying and solvent recovery during lithium-ion battery manufacturing. J. Power Sources 322, 169–178 (2016).
Susarla, N., Ahmed, S. & Dees, D. W. Modeling and analysis of solvent removal during Li-ion battery electrode drying. J. Power Sources 378, 660–670 (2018).
Stein, M., Mistry, A. & Mukherjee, P. P. Mechanistic understanding of the role of evaporation in electrode processing. J. Electrochem. Soc. 164, A1616 (2017).
Polizos, G. et al. Two-layer cathode architecture for high-energy density and high-power density solid state batteries. Mater. Today Chem. 33, 101704 (2023).
Parikh, D. & Li, J. Bilayer hybrid graphite anodes via freeze tape casting for extreme fast charging applications. Carbon 196, 525–531 (2022).
Tao, R. et al. High-throughput and high-performance lithium-ion batteries via dry processing. Chem. Eng. J. 471, 144300 (2023).
Arnold, J., Voelker, G., Fasolo, J. & Laden, P. UV or EB cured polymer-bonded ceramic particle lithium secondary battery separators, method for the production thereof. US Patent US10818900B2 (2020).
Pei, M. et al. 3D printing of advanced lithium batteries: a designing strategy of electrode/electrolyte architectures. J. Mater. Chem. A 9, 25237–25257 (2021).
Ank, M. et al. Lithium-ion cells in automotive applications: Tesla 4680 cylindrical cell teardown and characterization. J. Electrochem. Soc. 170, 120536 (2023).
Wood, D. L. III et al. Perspectives on the relationship between materials chemistry and roll-to-roll electrode manufacturing for high-energy lithium-ion batteries. Energy Storage Mater. 29, 254–265 (2020).
Li, J., Daniel, C. & Wood, D. Materials processing for lithium-ion batteries. J. Power Sources 196, 2452–2460 (2011).
Sherwood, J., Farmer, T. J. & Clark, J. H. Catalyst: possible consequences of the N-methyl pyrrolidone REACH restriction. Chem 4, 2010–2012 (2018).
Wang, M., Dong, X., Escobar, I. C. & Cheng, Y.-T. Lithium ion battery electrodes made using dimethyl sulfoxide (DMSO)—a green solvent. ACS Sustain. Chem. Eng. 8, 11046–11051 (2020).
Zhou, H., Pei, B., Fan, Q., Xin, F. & Whittingham, M. S. Can greener cyrene replace NMP for electrode preparation of NMC 811 cathodes? J. Electrochem. Soc. 168, 040536 (2021).
Ravikumar, V. R. et al. γ-Valerolactone: an alternative solvent for manufacturing of lithium-ion battery electrodes. ACS Appl. Energy Mater. 4, 696–703 (2021).
Sarkar, A., May, R., Valmonte, Z. & Marbella, L. E. PolarClean & dimethyl isosorbide: green matches in formulating cathode slurry. Energy Adv. 1, 671–676 (2022).
Li, J., Armstrong, B. L., Daniel, C., Kiggans, J. & Wood, D. L. III Optimization of multicomponent aqueous suspensions of lithium iron phosphate (LiFePO4) nanoparticles and carbon black for lithium-ion battery cathodes. J. Colloid Interface Sci. 405, 118–124 (2013).
Du, Z. et al. Enabling aqueous processing for crack-free thick electrodes. J. Power Sources 354, 200–206 (2017).
Sliz, R. et al. Suitable cathode NMP replacement for efficient sustainable printed Li-ion batteries. ACS Appl. Energy Mater. 5, 4047–4058 (2022).
Wood, M. et al. Chemical stability and long-term cell performance of low-cobalt, Ni-rich cathodes prepared by aqueous processing for high-energy Li-ion batteries. Energy Storage Mater. 24, 188–197 (2020).
Hofmann, M., Nagler, F., Kapuschinski, M., Guntow, U. & Giffin, G. A. Surface modification of LiNi0.8Co0.15Al0.05O2 particles via Li3PO4 coating to enable aqueous electrode processing. ChemSusChem 13, 5962–5971 (2020).
Hawley, W. B. & Li, J. Electrode manufacturing for lithium-ion batteries—analysis of current and next generation processing. J. Energy Storage 25, 100862 (2019).
Li, C.-C. & Lin, Y.-S. Interactions between organic additives and active powders in water-based lithium iron phosphate electrode slurries. J. Power Sources 220, 413–421 (2012).
Chai, L. et al. Chitosan, a new and environmental benign electrode binder for use with graphite anode in lithium-ion batteries. Electrochim. Acta 105, 378–383 (2013).
Kovalenko, I. et al. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 334, 75–79 (2011).
Li, J., Armstrong, B. L., Kiggans, J., Daniel, C. & Wood, D. L. III Optimization of LiFePO4 nanoparticle suspensions with polyethyleneimine for aqueous processing. Langmuir 28, 3783–3790 (2012).
Versaci, D. et al. New eco-friendly low-cost binders for Li-ion anodes. J. Solid State Electrochem. 21, 3429–3435 (2017).
Kalnaus, S., Livingston, K., Hawley, W. B., Wang, H. & Li, J. Design and processing for high performance Li ion battery electrodes with double-layer structure. J. Energy Storage 44, 103582 (2021).
Sahore, R. et al. Performance of different water-based binder formulations for Ni-rich cathodes evaluated in LiNi0.8Mn0.1Co0.1O2//graphite pouch cells. J. Electrochem. Soc. 169, 040567 (2022).
Reissig, F. et al. Investigation of lithium polyacrylate binders for aqueous processing of Ni-rich lithium layered oxide cathodes for lithium-ion batteries. ChemSusChem 15, e202200401 (2022).
Hays, K. A. et al. What makes lithium substituted polyacrylic acid a better binder than polyacrylic acid for silicon–graphite composite anodes? J. Power Sources 384, 136–144 (2018).
Uzun, K. et al. Investigating the structure and performance of electrodes made by dry and wet slurry processes. J. Electrochem. Soc. 171, 020516 (2024).
Boz, B. et al. Evaluating polyacrylic acid as a universal aqueous binder for Ni-rich cathodes NMC811 and Si anodes in full cell lithium-ion batteries. ChemPlusChem 89, e202400195 (2024).
Aguiló-Aguayo, N., Hubmann, D., Khan, F. U., Arzbacher, S. & Bechtold, T. Water-based slurries for high-energy LiFePO4 batteries using embroidered current collectors. Sci. Rep. 10, 5565 (2020).
Surace, Y., Jahn, M. & Cupid, D. M. The rate capability performance of high-areal-capacity water-based NMC811 electrodes: the role of binders and current collectors. Batteries 10, 100 (2024).
Porcher, W., Lestriez, B., Jouanneau, S. & Guyomard, D. Design of aqueous processed thick LiFePO4 composite electrodes for high-energy lithium battery. J. Electrochem. Soc. 156, A133 (2009).
Sahore, R. et al. Towards understanding of cracking during drying of thick aqueous-processed LiNi0.8Mn0.1Co0.1O2 cathodes. ACS Sustain. Chem. Eng. 8, 3162–3169 (2020).
Hawley, W. B. et al. Lithium and transition metal dissolution due to aqueous processing in lithium-ion battery cathode active materials. J. Power Sources 466, 228315 (2020).
Li, J. et al. Toward low-cost, high-energy density, and high-power density lithium-ion batteries. JOM 69, 1484–1496 (2017).
Loeffler, N. et al. In situ coating of Li[Ni0.33Mn0.33Co0.33]O2 particles to enable aqueous electrode processing. ChemSusChem 9, 1112–1117 (2016).
Sharma, J. et al. Enhancing the electrochemical performance of aqueous processed Li-ion cathodes with silicon oxide coatings. ChemSusChem 16, e202300350 (2023).
Nagler, F. et al. Influence of phosphate surface coating on performance of aqueous-processed NMC811 cathodes in 3 Ah lithium-ion cells. ChemElectroChem 11, e202300748 (2024).
Kukay, A. et al. Aqueous Ni-rich-cathode dispersions processed with phosphoric acid for lithium-ion batteries with ultra-thick electrodes. J. Colloid Interface Sci. 581, 635–643 (2021).
Kong, J. et al. Toward high-energy-density aqueous lithium-ion batteries using silver nanowires as current collectors. Molecules 27, 8207 (2022).
Li, J., Rulison, C., Kiggans, J., Daniel, C. & Wood, D. L. Superior performance of LiFePO4 aqueous dispersions via corona treatment and surface energy optimization. J. Electrochem. Soc. 159, A1152 (2012).
Neidhart, L., Fröhlich, K., Winter, F. & Jahn, M. Implementing binder gradients in thick water-based NMC811 cathodes via multi-layer coating. Batteries 9, 171 (2023).
Ibing, L. et al. Towards water based ultra-thick Li ion battery electrodes—a binder approach. J. Power Sources 423, 183–191 (2019).
Porcher, W., Lestriez, B., Jouanneau, S. & Guyomard, D. Optimizing the surfactant for the aqueous processing of LiFePO4 composite electrodes. J. Power Sources 195, 2835–2843 (2010).
Lee, J., Kumar, P., Lee, G., Moudgil, B. M. & Singh, R. K. Electrochemical performance of surfactant-processed LiFePO4 as a cathode material for lithium-ion rechargeable batteries. Ionics 19, 371–378 (2013).
Li, J., Daniel, C., An, S. J. & Wood, D. Evaluation residual moisture in lithium-ion battery electrodes and its effect on electrode performance. MRS Adv. 1, 1029–1035 (2016).
Hays, K. A., Key, B., Li, J., Wood, D. L. III & Veith, G. M. Si oxidation and H2 gassing during aqueous slurry preparation for Li-ion battery anodes. J. Phys. Chem. C 122, 9746–9754 (2018).
Reynolds, C. D., Slater, P. R., Hare, S. D., Simmons, M. J. H. & Kendrick, E. A review of metrology in lithium-ion electrode coating processes. Mater. Des. 209, 109971 (2021).
Hawley, W. B., Kays, W. & Li, J. in Processing and Manufacturing of Electrodes for Lithium-Ion Batteries Ch. 8 (eds Li, J. & Jin, C.) (Institution of Engineering and Technology, 2024).
Hawley, W. B. & Li, J. Beneficial rheological properties of lithium-ion battery cathode slurries from elevated mixing and coating temperatures. J. Energy Storage 26, 100994 (2019).
Parikh, D., Christensen, T. & Li, J. Correlating the influence of porosity, tortuosity, and mass loading on the energy density of LiNi0.6Mn0.2Co0.2O2 cathodes under extreme fast charging (XFC) conditions. J. Power Sources 474, 228601 (2020).
Du, Z. et al. Three-dimensional conductive network formed by carbon nanotubes in aqueous processed NMC electrode. Electrochim. Acta 270, 54–61 (2018).
Wood, M. et al. Impact of secondary particle size and two-layer architectures on the high-rate performance of thick electrodes in lithium-ion battery pouch cells. J. Power Sources 515, 230429 (2021).
Kang, H., Kim, Y., Yoon, T. & Mun, J. Improved performance of lithium-ion batteries using a multilayer cathode of LiFePO4 and LiNi0.8Co0.1Mn0.1O2. J. Electrochem. Sci. Technol. 14, 320–325 (2023).
Neidhart, L. et al. Aqueous manufacturing of defect-free thick multi-layer NMC811 electrodes. Nanomaterials 12, 317 (2022).
Yu, S., Kim, S., Kim, T. Y., Nam, J. H. & Cho, W. I. Model prediction and experiments for the electrode design optimization of LiFePO4/graphite electrodes in high capacity lithium-ion batteries. Bull. Korean Chem. Soc. 34, 79–88 (2013).
Li, J. & Jin, C. Processing and Manufacturing of Electrodes for Lithium-Ion Batteries (Institution of Engineering and Technology, 2023).
Zhu, P., Han, J. & Pfleging, W. Characterization and laser structuring of aqueous processed Li(Ni0.6Mn0.2Co0.2)O2 thick-film cathodes for lithium-ion batteries. Nanomaterials 11, 1840 (2021).
Cobb, C. L. & Blanco, M. Modeling mass and density distribution effects on the performance of co-extruded electrodes for high energy density lithium-ion batteries. J. Power Sources 249, 357–366 (2014).
Tao, R. et al. Freeze tape casting electrode with bilayered architecture for high-performance lithium-ion batteries. ACS Appl. Energy Mater. 7, 856–861 (2024).
Shodiev, A. et al. Designing electrode architectures to facilitate electrolyte infiltration for lithium-ion batteries. Energy Storage Mater. 49, 268–277 (2022).
Smyrek, P. & Pfleging, W. in Processing and Manufacturing of Electrodes for Lithium-Ion Batteries Energy Engineering (eds Li, J. & Jin, C.) 101–127 (Institution of Engineering and Technology, 2023).
Cobb, C. L. & Solberg, S. E. Communication—analysis of thick co-extruded cathodes for higher-energy-and-power lithium-ion batteries. J. Electrochem. Soc. 164, A1339 (2017).
Pfleging, W. A review of laser electrode processing for development and manufacturing of lithium-ion batteries. Nanophotonics 7, 549–573 (2018).
Naguib, M. et al. Limiting internal short-circuit damage by electrode partition for impact-tolerant Li-ion batteries. Joule 2, 155–167 (2018).
Kalnaus, S. et al. Multifunctional approaches for safe structural batteries. J. Energy Storage 40, 102747 (2021).
Li, J. et al. Water-based electrode manufacturing and direct recycling of lithium-ion battery electrodes—a green and sustainable manufacturing system. iScience 23, 101081 (2020).
Mohanty, D. et al. Non-destructive evaluation of slot-die-coated lithium secondary battery electrodes by in-line laser caliper and IR thermography methods. Anal. Methods 6, 674–683 (2014).
Qu, Z. et al. Thermal-assisted dry coating electrode unlocking sustainable and high-performance batteries. Adv. Mater. https://doi.org/10.1002/adma.202410974 (2024).
Kletz, T. A. Inherently safer design: the growth of an idea. Process. Saf. Prog. 15, 5–8 (1996).
Kletz, T. A. & Amyotte, P. Process Plants: A Handbook for Inherently Safer Design (CRC, 2010).
Tao, R. et al. Unraveling the impact of the degree of dry mixing on dry-processed lithium-ion battery electrodes. J. Power Sources 580, 233379 (2023).
Bouguern, M. D. et al. Engineering dry electrode manufacturing for sustainable lithium-ion batteries. Batteries 10, 39 (2024).
Degen, F. & Krätzig, O. Future in battery production: an extensive benchmarking of novel production technologies as guidance for decision making in engineering. IEEE Trans. Eng. Manag. 71, 1038–1056 (2024).
Liu, Y. et al. Roll-to-roll solvent-free manufactured electrodes for fast-charging batteries. Joule 7, 952–970 (2023).
Lu, Y. et al. Dry electrode technology, the rising star in solid-state battery industrialization. Matter 5, 876–898 (2022).
Gao, Z. et al. Particle interactions during dry powder mixing and their effect on solvent-free manufactured electrode properties. J. Energy Storage 83, 110605 (2024).
Li, B., Sosik, R. & Wixom, M. Compositions and methods for electro-chemical cell component fabrication. US Patent US20230076834A1 (2023).
Bockholt, H., Haselrieder, W. & Kwade, A. Intensive powder mixing for dry dispersing of carbon black and its relevance for lithium-ion battery cathodes. Powder Technol. 297, 266–274 (2016).
Wang, M. et al. Influence of mixing process on the performance of electrodes made by a dry coating method. J. Electrochem. Soc. 170, 010541 (2023).
Gyulai, A., Bauer, W. & Ehrenberg, H. Dry electrode manufacturing in a calender: the role of powder premixing for electrode quality and electrochemical performance. ACS Appl. Energy Mater. 6, 5122–5134 (2023).
Ludwig, B. et al. Understanding interfacial-energy-driven dry powder mixing for solvent-free additive manufacturing of Li-ion battery electrodes. Adv. Mater. Interfaces 4, 1700570 (2017).
Bruckner, J., Tschocke, S., Althues, H., Kaskel, S. & Thieme, S. Cathode for lithium-containing batteries and solvent-free method for the production thereof. US Patent 10,062,900 (2018).
Yang, G. et al. Robust solid/electrolyte interphase (SEI) formation on Si anodes using glyme-based electrolytes. ACS Energy Lett. 6, 1684–1693 (2021).
Zhang, W. et al. In-situ constructing hetero-structured Mo2C–Mo2N embedded in carbon nanosheet as an efficient separator modifier for high-performance lithium-sulfur batteries. Chem. Eng. J. 475, 146133 (2023).
Fiedler, M. et al. Mechanistic insights into the cycling behavior of sulfur dry-film cathodes. Adv. Sustain. Syst. 7, 2200439 (2023).
Verdier, N. et al. Challenges in solvent-free methods for manufacturing electrodes and electrolytes for lithium-based batteries. Polymers 13, 323 (2021).
Oh, H. et al. Development of a feasible and scalable manufacturing method for PTFE-based solvent-free lithium-ion battery electrodes. Chem. Eng. J. 491, 151957 (2024).
Furukawa, G. T., McCoskey, R. E. & King, G. J. Calorimetric properties of polytetrafluoroethylene (Teflon) from 0 to 365 K. J. Res. Natl. Bur. Stand. 49, 273–276 (1952).
Noda, Y., Hosokawa, K., Mizuno, T., Ihara, K. & Shimizu, T. Polytetrafluoroethylene fine particles and powder. US Patent 5,324,785 (1994).
Li, Y. et al. Progress in solvent-free dry-film technology for batteries and supercapacitors. Mater. Today 55, 92–109 (2022).
Yao, W. et al. A 5 V-class cobalt-free battery cathode with high loading enabled by dry coating. Energy Environ. Sci. 16, 1620–1630 (2023).
Tao, R. et al. Correlation among porosity, mechanical properties, morphology, electronic conductivity and electrochemical kinetics of dry-processed electrodes. J. Power Sources 581, 233481 (2023).
Moiseev, I. A. et al. Single crystal Ni-rich NMC cathode materials for lithium-ion batteries with ultra-high volumetric energy density. Energy Adv. 1, 677–681 (2022).
Min, J., Suk, W., Wong, S. C. Y. & Li, Y. Single-particle electrochemical cycling single-crystal and polycrystalline NMC particles. Adv. Funct. Mater. 34, 2410241 (2024).
Bi, Y. et al. Simultaneous single crystal growth and segregation of Ni-rich cathode enabled by nanoscale phase separation for advanced lithium-ion batteries. Energy Storage Mater. 62, 102947 (2023).
Tao, R. et al. Exploring the potential and impact of single-crystal active materials on dry-processed electrodes for high-performance lithium-ion batteries. Chem. Eng. J. 500, 157194 (2024).
Tao, R. et al. Insights into the chemistry of the cathodic electrolyte interphase for PTFE-based dry-processed cathodes. ACS Appl. Mater. Interfaces 15, 40488–40495 (2023).
Liu, W. et al. Electrochemical and X-ray photospectroscopy studies of polytetrafluoroethylene and polyvinylidene fluoride in Li/C batteries. J. Power Sources 68, 344–347 (1997).
Li, G., Xue, R. & Chen, L. The influence of polytetrafluorethylene reduction on the capacity loss of the carbon anode for lithium ion batteries. Solid. State Ion. 90, 221–225 (1996).
Wei, Z. et al. Removing electrochemical constraints on polytetrafluoroethylene as dry-process binder for high-loading graphite anodes. Joule 8, 1350–1363 (2024).
Lee, T. et al. Non-electroconductive polymer coating on graphite mitigating electrochemical degradation of PTFE for a dry-processed lithium-ion battery anode. ACS Appl. Mater. Interfaces 16, 8930–8938 (2024).
Zhang, Y. et al. Revisiting polytetrafluorethylene binder for solvent-free lithium-ion battery anode fabrication. Batteries 8, 57 (2022).
Zhang, Y., Lu, S., Lou, F. & Yu, Z. Leveraging synergies by combining polytetrafluorethylene with polyvinylidene fluoride for solvent-free graphite anode fabrication. Energy Technol. 10, 2200732 (2022).
Ryu, M., Hong, Y.-K., Lee, S.-Y. & Park, J. H. Ultrahigh loading dry-process for solvent-free lithium-ion battery electrode fabrication. Nat. Commun. 14, 1316 (2023).
Helmers, L. et al. Sustainable solvent-free production and resulting performance of polymer electrolyte-based all-solid-state battery electrodes. Energy Technol. 9, 2000923 (2021).
de la Torre-Gamarra, C. et al. High mass loading additive-free LiFePO4 cathodes with 500 μm thickness for high areal capacity Li-ion batteries. J. Power Sources 458, 228033 (2020).
Yuan, L., Liu, H. & Jiang, X. Employing polyaniline conductive binders for graphite lithium-ion anodes via a dry process. J. Energy Storage 90, 111912 (2024).
Wang, J. et al. High-area-capacity cathode by ultralong carbon nanotubes for secondary binder-assisted dry coating technology. ACS Appl. Mater. Interfaces 16, 26209–26216 (2024).
Park, D.-W., Cañas, N. A., Wagner, N. & Friedrich, K. A. Novel solvent-free direct coating process for battery electrodes and their electrochemical performance. J. Power Sources 306, 758–763 (2016).
Uzun, K. et al. Investigating the effect of electrode compositions on dry-made NMC811 positive electrodes. J. Electrochem. Soc. 171, 080532 (2024).
Chen, Z., Rana, D., Matsuura, T., Meng, D. & Lan, C. Q. Study on structure and vacuum membrane distillation performance of PVDF membranes: II. Influence of molecular weight. Chem. Eng. J. 276, 174–184 (2015).
Wang, M., Hu, J., Wang, Y. & Cheng, Y.-T. The influence of polyvinylidene fluoride (PVDF) binder properties on LiNi0.33Co0.33Mn0.33O2 (NMC) electrodes made by a dry-powder-coating process. J. Electrochem. Soc. 166, A2151 (2019).
Zhen, E. et al. Effects of binder content on low-cost solvent-free electrodes made by dry-spraying manufacturing for lithium-ion batteries. J. Power Sources 515, 230644 (2021).
Al-Shroofy, M. et al. Solvent-free dry powder coating process for low-cost manufacturing of LiNi1/3Mn1/3Co1/3O2 cathodes in lithium-ion batteries. J. Power Sources 352, 187–193 (2017).
Liu, J. et al. Scalable dry printing manufacturing to enable long-life and high energy lithium-ion batteries. Adv. Mater. Technol. 2, 1700106 (2017).
Shiraki, S. et al. Fabrication of all-solid-state battery using epitaxial LiCoO2 thin films. J. Power Sources 267, 881–887 (2014).
Reyes Jiménez, A. et al. A step toward high-energy silicon-based thin film lithium ion batteries. ACS Nano 11, 4731–4744 (2017).
Bates, J. B., Dudney, N. J., Neudecker, B., Ueda, A. & Evans, C. D. Thin-film lithium and lithium-ion batteries. Solid. State Ion. 135, 33–45 (2000).
Kirsch, D. J. et al. Scalable dry processing of binder-free lithium-ion battery electrodes enabled by holey graphene. ACS Appl. Energy Mater. 2, 2990–2997 (2019).
Gazda, J. et al. Advanced lithium (Li) ion and lithium sulfur (LiS) batteries. US Patent US11133495B2 (2021).
Lanning, B., Stowell, M. W., Gazda, J. & Bell, J. 3D self-assembled multi-modal carbon-based particle. US Patent 11,198,611 (2021).
Tao, R. et al. In-situ ionothermal synthesis of nanoporous carbon/oxide composites: a new key to functional separators for stable lithium–sulfur batteries. Nano Energy 130, 110091 (2024).
Hippauf, F. et al. Overcoming binder limitations of sheet-type solid-state cathodes using a solvent-free dry-film approach. Energy Storage Mater. 21, 390–398 (2019).
Wang, C. et al. Solvent-free approach for interweaving freestanding and ultrathin inorganic solid electrolyte membranes. ACS Energy Lett. 7, 410–416 (2022).
Zhang, P., Wixom, M. & Sosik, R. Dry process formation of solid state lithium ion cell. US Patent 11,870,057 (2024).
Tan, D. H. S., Meng, Y. S. & Jang, J. Scaling up high-energy-density sulfidic solid-state batteries: a lab-to-pilot perspective. Joule 6, 1755–1769 (2022).
Sul, H., Lee, D. & Manthiram, A. High-loading lithium–sulfur batteries with solvent-free dry-electrode processing. Small 20, 2400728 (2024).
Hu, J.-K. et al. Dry electrode technology for scalable and flexible high-energy sulfur cathodes in all-solid-state lithium–sulfur batteries. J. Energy Chem. 71, 612–618 (2022).
Schwalm, R. UV Coatings: Basics, Recent Developments and New Applications (Elsevier, 2007).
Du, Z., Janke, C. J., Li, J., Daniel, C. & Wood, D. Electron beam curing of composite positive electrode for Li-ion battery. J. Electrochem. Soc. 163, A2776 (2016).
Du, Z., Janke, C. J., Li, J. & Wood, D. L. High–speed electron beam curing of thick electrode for high energy density Li-ion batteries. Green. Energy Environ. 4, 375–381 (2019).
Xue, Z., Hu, L., Amine, K. & Zhang, Z. High-speed fabrication of lithium-ion battery electrodes by UV-curing. Energy Technol. 3, 469–475 (2015).
Voelker, G. & Arnold, D. J. Development of Ultraviolet Curable Binder Technology to Reduce Manufacturing Cost and Improve Performance of Lithium ion Battery Electrodes (Miltec UV, 2019).
Voelker, G. & Arnold, J. Dramatically Improve the Safety Performance of Li Ion Battery Separators and Reduce the Manufacturing Cost Using Ultraviolet Curing and High Precision Coating Technologies (Miltec UV, 2017).
Hoyle, C. E. & Kinstle, J. F. Radiation Curing of Polymeric Materials (eds Hoyle, C. E. & Kinstle, J. F.) Vol. 417 (American Chemical Society, 1990).
Abou Elmaaty, T., Okubayashi, S., Elsisi, H. & Abouelenin, S. Electron beam irradiation treatment of textiles materials: a review. J. Polym. Res. 29, 117 (2022).
Du, Z., Wood, D. L., Daniel, C., Kalnaus, S. & Li, J. Understanding limiting factors in thick electrode performance as applied to high energy density Li-ion batteries. J. Appl. Electrochem. 47, 405–415 (2017).
Je, M. et al. Formulating electron beam-induced covalent linkages for stable and high-energy-density silicon microparticle anode. Adv. Sci. 11, 2305298 (2024).
Zhou, L. et al. 3D-printed microelectrodes with a developed conductive network and hierarchical pores toward high areal capacity for microbatteries. Adv. Mater. Technol. 4, 1800402 (2019).
Li, J., Leu, M. C., Panat, R. & Park, J. A hybrid three-dimensionally structured electrode for lithium-ion batteries via 3D printing. Mater. Des. 119, 417–424 (2017).
Praveen, S., Santhoshkumar, P., Joe, Y. C., Senthil, C. & Lee, C. W. 3D-printed architecture of Li-ion batteries and its applications to smart wearable electronic devices. Appl. Mater. Today 20, 100688 (2020).
Ao, S., Guo, Z., Song, Y., Fang, D. & Bao, Y. Clog-free, low-cost, and uniform electrode inks for 3D printed lithium-ion batteries. ACS Appl. Energy Mater. 5, 6970–6979 (2022).
Saleh, M. S., Li, J., Park, J. & Panat, R. 3D printed hierarchically-porous microlattice electrode materials for exceptionally high specific capacity and areal capacity lithium ion batteries. Addit. Manuf. 23, 70–78 (2018).
Praveen, S., Sim, G. S., Ho, C. W. & Lee, C. W. 3D-printed twisted yarn-type Li-ion battery towards smart fabrics. Energy Stor. Mater. 41, 748–757 (2021).
Pang, Y. et al. Additive manufacturing of batteries. Adv. Funct. Mater. 30, 1906244 (2020).
Yan, J. et al. Direct-ink writing 3D printed energy storage devices: from material selectivity, design and optimization strategies to diverse applications. Mater. Today 54, 110–152 (2022).
Yang, Y. et al. Overview on the applications of three-dimensional printing for rechargeable lithium-ion batteries. Appl. Energy 257, 114002 (2020).
Sun, C. et al. 3D printing nanocomposite gel-based thick electrode enabling both high areal capacity and rate performance for lithium-ion battery. Chem. Eng. J. 381, 122641 (2020).
Tao, R., Gu, Y., Sharma, J., Hong, K. & Li, J. A conformal heat-drying direct ink writing 3D printing for high-performance lithium-ion batteries. Mater. Today Chem. 32, 101672 (2023).
Bao, Y., Liu, Y., Kuang, Y., Fang, D. & Li, T. 3D-printed highly deformable electrodes for flexible lithium ion batteries. Energy Storage Mater. 33, 55–61 (2020).
Sun, K. et al. 3D printing of interdigitated Li-ion microbattery architectures. Adv. Mater. 25, 4539–4543 (2013).
Yang, P. & Fan, H. J. Inkjet and extrusion printing for electrochemical energy storage: a minireview. Adv. Mater. Technol. 5, 2000217 (2020).
Foresti, D. et al. Acoustophoretic printing. Sci. Adv. 4, eaat1659 (2018).
Delannoy, P. E. et al. Ink-jet printed porous composite LiFePO4 electrode from aqueous suspension for microbatteries. J. Power Sources 287, 261–268 (2015).
Mosa, M. A., Jo, J. Y. & Kwon, K.-S. Fast on-off jet control of aerosol jet printing (AJP) using internal rotary valve. Addit. Manuf. 67, 103466 (2023).
Deiner, L. J., Jenkins, T., Powell, A., Howell, T. & Rottmayer, M. High capacity rate capable aerosol jet printed Li-ion battery cathode. Adv. Eng. Mater. 21, 1801281 (2019).
Flowers, P. F., Reyes, C., Ye, S., Kim, M. J. & Wiley, B. J. 3D printing electronic components and circuits with conductive thermoplastic filament. Addit. Manuf. 18, 156–163 (2017).
Zhang, W. et al. 3D printed micro-electrochemical energy storage devices: from design to integration. Adv. Funct. Mater. 31, 2104909 (2021).
Mishra, V., Negi, S. & Kar, S. FDM-based additive manufacturing of recycled thermoplastics and associated composites. J. Mater. Cycles Waste Manag. 25, 758–784 (2023).
Foster, C. W. et al. Next-generation additive manufacturing: tailorable graphene/polylactic(acid) filaments allow the fabrication of 3D printable porous anodes for utilisation within lithium-ion batteries. Batteries Supercaps 2, 448–453 (2019).
Maurel, A. et al. Highly loaded graphite–polylactic acid composite-based filaments for lithium-ion battery three-dimensional printing. Chem. Mater. 30, 7484–7493 (2018).
Maines, E. M., Porwal, M. K., Ellison, C. J. & Reineke, T. M. Sustainable advances in SLA/DLP 3D printing materials and processes. Green. Chem. 23, 6863–6897 (2021).
Salas, A., Zanatta, M., Sans, V. & Roppolo, I. Chemistry in light-induced 3D printing. ChemTexts 9, 4 (2023).
Katsuyama, Y. et al. A 3D-printed, freestanding carbon lattice for sodium ion batteries. Small 18, 2202277 (2022).
Pikul, J. H., Gang Zhang, H., Cho, J., Braun, P. V. & King, W. P. High-power lithium ion microbatteries from interdigitated three-dimensional bicontinuous nanoporous electrodes. Nat. Commun. 4, 1732 (2013).
Duffner, F. et al. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 6, 123–134 (2021).
Janek, J. & Zeier, W. G. Challenges in speeding up solid-state battery development. Nat. Energy 8, 230–240 (2023).
Hawley, W. B. et al. Deconvoluting sources of failure in lithium metal batteries containing NMC and PEO-based electrolytes. Electrochim. Acta 404, 139579 (2022).
Sahore, R. et al. A bilayer electrolyte design to enable high-areal-capacity composite cathodes in polymer electrolytes based solid-state lithium metal batteries. ACS Appl. Energy Mater. 5, 1409–1413 (2022).
Kalnaus, S., Dudney, N. J., Westover, A. S., Herbert, E. & Hackney, S. Solid-state batteries: the critical role of mechanics. Science 381, eabg5998 (2023).
Wang, C. et al. Boosting the performance of lithium batteries with solid–liquid hybrid electrolytes: interfacial properties and effects of liquid electrolytes. Nano Energy 48, 35–43 (2018).
Lv, C. et al. Machine learning: an advanced platform for materials development and state prediction in lithium-ion batteries. Adv. Mater. 34, 2101474 (2022).
Chan, C. H., Sun, M. & Huang, B. Application of machine learning for advanced material prediction and design. EcoMat 4, e12194 (2022).
Reynolds, C. et al. Impact of formulation and slurry properties on lithium-ion electrode manufacturing. Batteries Supercaps 7, e202300396 (2024).
Faraji Niri, M., Reynolds, C., Román Ramírez, L. A. A., Kendrick, E. & Marco, J. Systematic analysis of the impact of slurry coating on manufacture of Li-ion battery electrodes via explainable machine learning. Energy Storage Mater. 51, 223–238 (2022).
Duquesnoy, M., Lombardo, T., Chouchane, M., Primo, E. N. & Franco, A. A. Data-driven assessment of electrode calendering process by combining experimental results, in silico mesostructures generation and machine learning. J. Power Sources 480, 229103 (2020).
Shodiev, A. et al. Machine learning 3D-resolved prediction of electrolyte infiltration in battery porous electrodes. J. Power Sources 511, 230384 (2021).
Diddens, D. et al. Modeling the solid electrolyte interphase: machine learning as a game changer? Adv. Mater. Interfaces 9, 2101734 (2022).
Rauf, H., Khalid, M. & Arshad, N. Machine learning in state of health and remaining useful life estimation: theoretical and technological development in battery degradation modelling. Renew. Sustain. Energy Rev. 156, 111903 (2022).
Lombardo, T. et al. Artificial intelligence applied to battery research: hype or reality? Chem. Rev. 122, 10899–10969 (2022).
Wu, X. et al. Progress, key issues, and future prospects for Li-ion battery recycling. Glob. Chall. 6, 2200067 (2022).
Wu, J. et al. Direct recovery: a sustainable recycling technology for spent lithium-ion battery. Energy Storage Mater. 54, 120–134 (2023).
Bai, Y., Essehli, R., Jafta, C. J., Livingston, K. M. & Belharouak, I. Recovery of cathode materials and aluminum foil using a green solvent. ACS Sustain. Chem. Eng. 9, 6048–6055 (2021).
Wang, M., Tan, Q., Liu, L. & Li, J. A facile, environmentally friendly, and low-temperature approach for decomposition of polyvinylidene fluoride from the cathode electrode of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 7, 12799–12806 (2019).
Bai, Y., Muralidharan, N., Li, J., Essehli, R. & Belharouak, I. Sustainable direct recycling of lithium-ion batteries via solvent recovery of electrode materials. ChemSusChem 13, 5664–5670 (2020).
Zhang, X., Xie, Y., Cao, H., Nawaz, F. & Zhang, Y. A novel process for recycling and resynthesizing LiNi1/3Co1/3Mn1/3O2 from the cathode scraps intended for lithium-ion batteries. Waste Manag. 34, 1715–1724 (2014).
Sajid, M. & Ilyas, M. PTFE-coated non-stick cookware and toxicity concerns: a perspective. Environ. Sci. Pollut. Res. 24, 23436–23440 (2017).
Purser, D. A. Recent developments in understanding the toxicity of PTFE thermal decomposition products. Fire Mater. 16, 67–75 (1992).
Fang, Z. et al. The role of surface oxygen in eliminating fluorine impurities and relithiation toward direct cathode recycling. ACS Appl. Energy Mater. 7, 8943–8953 (2024).
Du, K. et al. In-situ synthesis of porous metal fluoride@carbon composite via simultaneous etching/fluorination enabled superior Li storage performance. Nano Energy 103, 107862 (2022).
Park, J. S., Kim, T. & Kim, W. S. Conductive cellulose composites with low percolation threshold for 3D printed electronics. Sci. Rep. 7, 3246 (2017).
Xu, F., Wang, T., Li, W. & Jiang, Z. Preparing ultra-thin nano-MnO2 electrodes using computer jet-printing method. Chem. Phys. Lett. 375, 247–251 (2003).
Sanumi, O. J., Ndungu, P. G. & Oboirien, B. O. Challenges of 3D printing in LIB electrodes: emphasis on material-design properties, and performance of 3D printed Si-based LIB electrodes. J. Power Sources 543, 231840 (2022).
Acknowledgements
The submitted manuscript was created by UChicago Argonne, LLC, Operator of Argonne National Laboratory (‘Argonne’). Argonne, a US Department of Energy (DOE) Office of Science laboratory, is operated under Contract DE-AC02-06CH11357. Part of the work was performed at Oak Ridge National Laboratory, managed by UT Battelle, LLC, for the US DOE under contract DE-AC05-00OR22725. R.T. and J.L. thank the US DOE Advanced Materials & Manufacturing Technology Office and Vehicle Technologies Office (VTO) for support. Z.D. thanks the sponsorship of VTO. Y.G. was funded by the National Science Foundation (NSF 21-013), which was granted to C. Yuan, Case Western Reserve University, and is supplementary funding for Award no. 2101129 from the Division of Chemical, Bioengineering, Environmental, and Transport Systems. The US Government retains and the publisher, by accepting the article for publication, acknowledges that the US Government retains a non-exclusive, paid-up, irrevocable, worldwide licence to publish or reproduce the published form of this manuscript, or allow others to do so, for US Government purposes. The DOE will provide public access to these results of federally sponsored research under the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Author information
Authors and Affiliations
Contributions
All authors researched data and wrote the article. R.T. and J.L. contributed substantially to the coordination and supervision. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
J.L. and Z.D. are inventors on issued US Patents 11,289,689 and 11,984,577 for method of solvent-free manufacturing of composite electrodes incorporating radiation curable binders that are licensed to Ateois; they receive royalties for this invention. J.L. is an inventor on issued US Patents 8,845,768 and 9,527,044 for proton conducting membranes for hydrogen production and separation that are licensed to Redox Power Systems, and receives royalties for these inventions; is an inventor on issued US Patents 9,847,531 and 10,374,234 for current collectors for improved safety that are licensed to Soteria Battery Innovation Group, and receives loyalties for these inventions; is an inventor on US Patent 10,910,628 for fast formation cycling for rechargeable batteries and US Patent 11,362,333 for cobalt-free layered oxide cathodes that are licensed to SPARZ, and receives loyalties for these inventions; and is an inventor on US Patents 8,956,688 and 9,685,652 for aqueous processing of composite lithium-ion electrode material, US Patent 10,684,128 for batch and continuous methods for evaluating the physical and thermal properties of films, US Patent 11,065,719 for laser-interference surface preparation for enhanced coating adhesion, US Patent 11,791,477 for roll-to-roll SOFC manufacturing method and system, and US Patents 11,916,206, 11,996,557 and 12,068,472 for battery recycling. J.L. and Z.D. declare that they are inventors on US Patent 10,601,027 for manufacturing of thick composite electrode using solvent mixtures. R.T., Y.G. and X.L. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clean Technology thanks Kun Fu, Sang-Young Lee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tao, R., Gu, Y., Du, Z. et al. Advanced electrode processing for lithium-ion battery manufacturing. Nat. Rev. Clean Technol. 1, 116–131 (2025). https://doi.org/10.1038/s44359-024-00018-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44359-024-00018-w
This article is cited by
-
Failure mechanisms and scalability of anode-free lithium-metal and lithium–sulfur batteries
Nature Reviews Clean Technology (2026)
-
Next-generation anodes for high-energy and low-cost sodium-ion batteries
Nature Reviews Materials (2026)
-
Advances and challenges in dry electrode process for solid-state batteries
Journal of Solid State Electrochemistry (2026)
-
Advanced direct recycling enables upcycling of spent lithium-ion batteries
Science China Chemistry (2026)
-
Dry-coating electrode fabrication for sustainable, high-performance batteries
Science China Chemistry (2025)