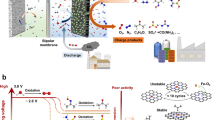
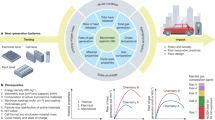
Abstract
By integrating energy storage with carbon dioxide (CO2) utilization, metal–CO2 batteries can contribute to net-zero energy storage and carbon management. However, challenges related to performance, cost and safety continue to hinder their commercial deployment. In this Review, we discuss two types of metal–CO2 battery — aqueous and non-aqueous — and examine their fundamental mechanisms, key component features and potential applications. In both systems, bifunctional catalysts and gas-diffusion layers are critical for efficient cathode reactions. Electrolyte design in non-aqueous systems must enable robust formation of the solid electrolyte interphase and broaden the operating temperature range (–120 °C to 50 °C). Aqueous systems need to suppress hydrogen evolution and carbonate formation effectively to ensure efficient CO2 reduction. Improving anode and separator stability, as well as optimizing cell configuration to ensure safe operation, are essential for long-term battery performance. Environmental and economic assessments reveal that most of the CO2 emissions and costs stem from cell components, requiring cost-efficient fabrication methods and recycling strategies to achieve sustainable and scalability targets. Future research should focus on integrated design principles that combine advanced materials, cell engineering and sustainability evaluation to unlock the full potential of metal–CO2 batteries as next-generation energy-storage and CO2-utilization technologies.
Key points
-
Metal–CO2 batteries offer both efficient energy storage and CO2 utilization, directly contributing to net-zero emissions goals.
-
The efficiency and stability of metal–CO2 batteries are influenced by electrode architecture, electrolyte formulation, anode engineering, separator optimization and gas purity.
-
Non-aqueous metal–CO2 batteries prioritize high energy storage, whereas aqueous systems offer a distinct advantage by converting CO2 into value-added chemicals during discharge.
-
Addressing safety issues, such as gas management, thermal runaway and electrolyte corrosion, is critical to enabling the large-scale deployment of metal-CO2 batteries.
-
The performance of metal–CO2 batteries is highly dependent on CO2 purity. Integration with CO2 capture systems and/or cathodes with enhanced impurity tolerance are needed.
-
Holistic development of metal–CO2 batteries, focusing on reducing CO2 emissions and costs in manufacturing, operating and recycling, could ensure long-term sustainability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jacobson, T. A. et al. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2, 691–701 (2019).
International Energy Agency. Global Energy Review 2021: Assessing the Effects of Economic Recoveries on Global Energy Demand and CO2 Emissions in 2021 (IEA, 2021).
Xie, Z., Zhang, X., Zhang, Z. & Zhou, Z. Metal–CO2 batteries on the road: CO2 from contamination gas to energy source. Adv. Mater. 29, 1605891 (2017).
Wang, F. et al. Metal–CO2 electrochemistry: from CO2 recycling to energy storage. Adv. Energy Mater. 11, 2100667 (2021).
Zhang, F. et al. Catalytic role of in-situ formed CN species for enhanced Li2CO3 decomposition. Nat. Commun. 15, 3393 (2024).
Zou, J. et al. Revisiting the role of discharge products in Li–CO2 batteries. Adv. Mater. 35, 2210671 (2023).
Gupta, D., Mao, J. & Guo, Z. Bifunctional catalysts for CO2 reduction and O2 evolution: a pivotal for aqueous rechargeable Zn–CO2 batteries. Adv. Mater. 36, 2407099 (2024).
Gupta, D., Zou, J., Mao, J. & Guo, Z. Concurrent energy storage and decarbonization by metal–CO2 batteries: aqueous or non-aqueous? Energy Environ. Sci. 18, 5215–5249 (2025).
Aslam, M. K., Wang, H., Chen, S., Li, Q. & Duan, J. Progress and perspectives of metal (Li, Na, Al, Zn and K)–CO2 batteries. Mater. Today Energy 31, 101196 (2023).
Li, W. et al. Boosting a practical Li–CO2 battery through dimerization reaction based on solid redox mediator. Nat. Commun. 15, 803 (2024).
Pathak, A. D., Adhikari, P. R. & Choi, W. Lithium–CO2 batteries and beyond. Front. Energy Res. 11, 1150737 (2023).
Burtt, D. G. et al. Highly enriched carbon and oxygen isotopes in carbonate-derived CO2 at Gale crater, Mars. Proc. Natl Acad. Sci. USA 121, e2321342121 (2024).
Ganesh, I. Electrochemical conversion of carbon dioxide into renewable fuel chemicals — the role of nanomaterials and the commercialization. Renew. Sustain. Energy Rev. 59, 1269–1297 (2016).
Lee, C.-Y., Zou, J. & Wallace, G. G. Electrochemical CO2 reduction and mineralisation in calcium containing electrolytes. Mater. Today Chem. 38, 102117 (2024).
Zou, J., Liang, G., Lee, C.-Y. & Wallace, G. G. Progress and perspectives for electrochemical CO2 reduction to formate. Mater. Today Energy 38, 101433 (2023).
Mu, X., He, P. & Zhou, H. Toward practical Li–CO2 batteries: mechanisms, catalysts, and perspectives. Acc. Mater. Res. 5, 467–478 (2024).
Xie, J. & Wang, Y. Recent development of CO2 electrochemistry from Li–CO2 batteries to Zn–CO2 batteries. Acc. Chem. Res. 52, 1721–1729 (2019).
Liu, W. et al. Advancements in metal–CO2 battery technology: a comprehensive overview. Nano Energy 129, 109998 (2024).
Suo, L. et al. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Bharti, A., Deb, D., Achutharao, G. & Bhattacharyya, A. J. CO2 crossover to the Li anode and its implications on the solid electrolyte interphase composition in a rechargeable Li–CO2 battery. J. Phys. Chem. C 128, 11543–11551 (2024).
Wang, Y. et al. Minireview on achieving low charge overpotential in Li–CO2 batteries with advancing cathode materials and electrolytes. Energy Fuels 38, 2743–2758 (2024).
Lu, Z. et al. A rechargeable Li–CO2 battery based on the preservation of dimethyl sulfoxide. J. Mater. Chem. A 10, 13821–13828 (2022).
Wang, L., Uosaki, K. & Noguchi, H. Effect of electrolyte concentration on the solvation structure of gold/LiTFSI–DMSO solution interface. J. Phys. Chem. C 124, 12381–12389 (2020).
Zhang, S. et al. Challenges and prospects of lithium–CO2 batteries. Nano Res. Energy 1, 9120001 (2022).
Lian, Z. et al. An integrated strategy for upgrading Li–CO2 batteries: redox mediator and separator modification. J. Chem. Eng. 450, 138400 (2022).
Jian, T. et al. From Ru to RuAl intermetallic/Ru heterojunction: enabling high reversibility of the CO2 redox reaction in Li–CO2 battery based on lowered interface thermodynamic energy barrier. Nano Energy 118, 108998 (2023).
Zhang, X. et al. Breaking the stable triangle of carbonate via W–O bonds for Li–CO2 batteries with low polarization. ACS Energy Lett. 6, 3503–3510 (2021).
Yang, S. et al. A reversible lithium–CO2 battery with Ru nanoparticles as a cathode catalyst. Energy Environ. Sci. 10, 972–978 (2017).
Wang, Y.-F., Ji, G.-J., Song, L.-N., Wang, X.-X. & Xu, J.-J. A highly reversible lithium–carbon dioxide battery based on soluble oxalate. ACS Energy Lett. 8, 1026–1034 (2023).
Pan, Q. et al. Approaching splendid catalysts for Li–CO2 battery from the theory to practical designing: a review. Adv. Mater. 36, 2406905 (2024).
Chourasia, A. K. et al. In situ/operando characterization techniques: the guiding tool for the development of Li–CO2 battery. Small Methods 6, 2200930 (2022).
Xie, J., Liu, Q., Huang, Y., Wu, M. & Wang, Y. A porous Zn cathode for Li–CO2 batteries generating fuel-gas CO. J. Mater. Chem. A 6, 13952–13958 (2018).
Zhang, W., Hu, C., Guo, Z. & Dai, L. High-performance K–CO2 batteries based on metal-free carbon electrocatalysts. Angew. Chem. Int. Ed. 59, 3470–3474 (2020).
Lin, J. et al. A comprehensive overview of the electrochemical mechanisms in emerging alkali metal-carbon dioxide batteries. Carbon Energy 5, e313 (2023).
Fetrow, C., Carugati, C., Yu, X., Zhou, X.-D. & Wei, S. A secondary Al–CO2 battery enabled by aluminum iodide as a homogeneous redox mediator. ACS Appl. Mater. Interfaces 15, 12908–12914 (2023).
Ma, W. et al. Rechargeable Al–CO2 batteries for reversible utilization of CO2. Adv. Mater. 30, 1801152 (2018).
Liu, W. et al. A nonaqueous Mg–CO2 battery with low overpotential. Adv. Energy Mater. 12, 2201675 (2022).
Kim, C. et al. Highly efficient CO2 utilization via aqueous zinc– or aluminum–CO2 systems for hydrogen gas evolution and electricity production. Angew. Chem. Int. Ed. 131, 9606–9611 (2019).
Deng, Y.-P. et al. The current state of aqueous Zn-based rechargeable batteries. ACS Energy Lett. 5, 1665–1675 (2020).
Ding, P. et al. Simultaneous power generation and CO2 valorization by aqueous Al–CO2 batteries using nanostructured Bi2S3 as the cathode electrocatalyst. J. Mater. Chem. A 8, 12385–12390 (2020).
Xie, J. et al. Reversible aqueous zinc–CO2 batteries based on CO2–HCOOH interconversion. Angew. Chem. Int. Ed. 57, 16996–17001 (2018).
Wang, Y. et al. Electron accumulation enables Bi efficient CO2 reduction for formate production to boost clean Zn–CO2 batteries. Nano Energy 92, 106780 (2022).
Noya, S., Uchida, M. & Yoshino, M. Double fluid cell. Japanese patent JPH04101358A (1990).
Thaller, L. H. Electrically rechargeable redox flow cell. US patent US3996064A (1976).
Sun, X. et al. A rechargeable “rocking chair” type Zn–CO2 battery. Angew. Chem. Int. Ed. 63, e202409977 (2024).
Sarkar, A. et al. Recent advances in rechargeable metal–CO2 batteries with nonaqueous electrolytes. Chem. Rev. 123, 9497–9564 (2023).
Kim, J. et al. Indirect surpassing CO2 utilization in membrane-free CO2 battery. Nano Energy 82, 105741 (2021).
Pathak, A. D., Adhikari, P. R. & Choi, W. High-efficiency rechargeable Fe–CO2 battery: a route for effective CO2 conversion and energy storage. ACS Appl. Mater. Interfaces 16, 21799–21806 (2024).
Xu, C., Dong, Y., Zhao, H. & Lei, Y. CO2 conversion toward real-world applications: electrocatalysis versus CO2 batteries. Adv. Funct. Mater. 33, 2300926 (2023).
van Deelen, T. W., Hernández Mejía, C. & de Jong, K. P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2, 955–970 (2019).
Lees, E. W., Mowbray, B. A., Parlane, F. G. & Berlinguette, C. P. Gas diffusion electrodes and membranes for CO2 reduction electrolysers. Nat. Rev. Mater. 7, 55–64 (2022).
Fetrow, C. J., Carugati, C., Zhou, X.-D. & Wei, S. Electrochemistry of metal–CO2 batteries: opportunities and challenges. Energy Storage Mater. 45, 911–933 (2022).
Lu, B. et al. Energy band engineering guided design of bidirectional catalyst for reversible Li–CO2 batteries. Adv. Mater. 36, 2308889 (2024).
Ooka, H., Huang, J. & Exner, K. S. The Sabatier principle in electrocatalysis: basics, limitations, and extensions. Front. Energy Res. 9, 654460 (2021).
Sun, L. et al. High entropy alloys enable durable and efficient lithium-mediated CO2 redox reactions. Adv. Mater. 36, 2401288 (2024).
Tseng, C.-M., Huang, C.-C., Pai, J.-Y. & Li, Y.-Y. Co single atom–FeCo alloy–carbon nanotube catalysts on graphene for lithium-oxygen and lithium-carbon dioxide batteries. ACS Sustain. Chem. Eng. 11, 8120–8130 (2023).
Wang, Z. et al. Dual catalytic sites of alloying effect bloom CO2 catalytic conversion for highly stable Li–CO2 battery. Adv. Funct. Mater. 33, 2213931 (2023).
Wang, X. et al. Rechargeable Zn–CO2 electrochemical cells mimicking two-step photosynthesis. Adv. Mater. 31, 1807807 (2019).
Giannakakis, G., Flytzani-Stephanopoulos, M. & Sykes, E. C. H. Single-atom alloys as a reductionist approach to the rational design of heterogeneous catalysts. Acc. Chem. Res. 52, 237–247 (2018).
Darby, M. T., Stamatakis, M., Michaelides, A. & Sykes, E. C. H. Lonely atoms with special gifts: breaking linear scaling relationships in heterogeneous catalysis with single-atom alloys. J. Phys. Chem. Lett. 9, 5636–5646 (2018).
Hannagan, R. T. et al. Investigating spillover energy as a descriptor for single-atom alloy catalyst design. J. Phys. Chem. Lett. 14, 10561–10569 (2023).
Zeng, Z. et al. Orbital coupling of hetero-diatomic nickel-iron site for bifunctional electrocatalysis of CO2 reduction and oxygen evolution. Nat. Commun. 12, 4088 (2021).
Chang, S.-Q., Cheng, C.-C., Cheng, P.-Y., Huang, C.-L. & Lu, S.-Y. Pulse electrodeposited FeCoNiMnW high entropy alloys as efficient and stable bifunctional electrocatalysts for acidic water splitting. J. Chem. Eng. 446, 137452 (2022).
Liu, S. et al. Defect-rich AuCu@Ag nanowires with exclusive strain effect accelerate nitrate reduction to ammonia. Appl. Catal. B 350, 123919 (2024).
Dąbrowa, J. et al. Demystifying the sluggish diffusion effect in high entropy alloys. J. Alloys Compd. 783, 193–207 (2019).
Lei, Y. et al. Carbon-supported high-entropy Co–Zn–Cd–Cu–Mn sulfide nanoarrays promise high-performance overall water splitting. Nano Res. 15, 6054–6061 (2022).
Li, H. et al. High-entropy alloy aerogels: a new platform for carbon dioxide reduction. Adv. Mater. 35, 2209242 (2023).
Zhang, T. et al. High-entropy alloy enables multi-path electron synergism and lattice oxygen activation for enhanced oxygen evolution activity. Nat. Commun. 16, 3327 (2025).
Zhu, K. et al. Single-atom cadmium-N4 sites for rechargeable Li–CO2 batteries with high capacity and ultra-long lifetime. Adv. Funct. Mater. 33, 2213841 (2023).
Han, L. et al. Stable and efficient single-atom Zn catalyst for CO2 reduction to CH4. J. Am. Chem. Soc. 142, 12563–12567 (2020).
Yang, R. et al. A trifunctional Ni–N/P–O-codoped graphene electrocatalyst enables dual-model rechargeable Zn–CO2/Zn–O2 batteries. J. Mater. Chem. A 7, 2575–2580 (2019).
Xu, C. et al. Multiscale defective interfaces for realizing Na–CO2 batteries with ultralong lifespan. Adv. Mater. 36, 2409533 (2024).
Chen, B. et al. Designing electrophilic and nucleophilic dual centers in the ReS2 plane toward efficient bifunctional catalysts for Li–CO2 batteries. J. Am. Chem. Soc. 144, 3106–3116 (2022).
Deng, H. et al. Substituent tuning of Cu coordination polymers enables carbon-efficient CO2 electroreduction to multi-carbon products. Nat. Commun. 15, 9706 (2024).
Yang, Y. et al. Ligand-tuning copper in coordination polymers for efficient electrochemical C–C coupling. Nat. Commun. 15, 6316 (2024).
Zheng, H. et al. Dispersed nickel phthalocyanine molecules on carbon nanotubes as cathode catalysts for Li–CO2 batteries. Small 19, 2302768 (2023).
Matsuda, S. et al. Cobalt phthalocyanine analogs as soluble catalysts that improve the charging performance of Li–O2 batteries. Chem. Phys. Lett. 620, 78–81 (2015).
Xu, S. et al. Immobilization of iron phthalocyanine on pyridine-functionalized carbon nanotubes for efficient nitrogen reduction reaction. ACS Catal. 12, 5502–5509 (2022).
Zou, J. et al. Size-dependent effects of Ru nanoparticles on Li–CO2 batteries. ACS Energy Lett. 9, 5145–5155 (2024).
Li, J. et al. High-efficiency and stable Li–CO2 battery enabled by carbon nanotube/carbon nitride heterostructured photocathode. Angew. Chem. Int. Ed. 134, e202114612 (2022).
Zhou, J. et al. A quasi-solid-state flexible fiber-shaped Li–CO2 battery with low overpotential and high energy efficiency. Adv. Mater. 31, 1804439 (2019).
Wu, C., Qi, G., Zhang, J., Cheng, J. & Wang, B. Porous Mo3P/Mo nanorods as efficient Mott–Schottky cathode catalysts for low polarization Li–CO2 battery. Small 19, 2302078 (2023).
Yang, C., Guo, K., Yuan, D., Cheng, J. & Wang, B. Unraveling reaction mechanisms of Mo2C as cathode catalyst in a Li–CO2 battery. J. Am. Chem. Soc. 142, 6983–6990 (2020).
Goodarzi, M., Nazari, F. & Illas, F. Assessing the performance of cobalt phthalocyanine nanoflakes as molecular catalysts for Li-promoted oxalate formation in Li–CO2–oxalate batteries. J. Phys. Chem. C 122, 25776–25784 (2018).
Sun, X. et al. Binuclear Cu complex catalysis enabling Li–CO2 battery with a high discharge voltage above 3.0 V. Nat. Commun. 14, 536 (2023).
Wang, A. et al. Imidazolium bromide based dual-functional redox mediator for the construction of dendrite-free Li-CO2 batteries. Chin. Chem. Lett. 36, 110186 (2024).
Wu, Y., Zhao, Z. & Peng, Z. Tailoring Li-CO2 electrochemistry based on 4,4′-bipyridine redox cycle. ACS Energy Lett. 8, 3430–3436 (2023).
Wang, L. et al. Optimizing CO2 reduction and evolution reaction mediated by o-phenylenediamine toward high performance Li–CO2 battery. Electrochim. Acta 419, 140424 (2022).
Xiao, X. et al. Achieving a high-specific-energy lithium-carbon dioxide battery by implementing a bi-side-diffusion structure. Appl. Energy 328, 120186 (2022).
Henckel, D. A. et al. Potential dependence of the local pH in a CO2 reduction electrolyzer. ACS Catal. 11, 255–263 (2020).
Dong, Y. & Komarneni, S. Strategies to develop Earth-abundant heterogeneous oxygen evolution reaction catalysts for pH-neutral or pH-near-neutral electrolytes. Small Methods 5, 2000719 (2021).
Nishimoto, T., Shinagawa, T., Naito, T. & Takanabe, K. Delivering the full potential of oxygen evolving electrocatalyst by conditioning electrolytes at near-neutral pH. ChemSusChem 14, 1554–1564 (2021).
Han, B. et al. Activity and stability trends of perovskite oxides for oxygen evolution catalysis at neutral pH. Phys. Chem. Chem. Phys. 17, 22576–22580 (2015).
Celorrio, V. N. et al. Strain effects on the oxidation of CO and HCOOH on Au–Pd core-shell nanoparticles. ACS Catal. 7, 1673–1680 (2017).
Gao, D. et al. Switchable CO2 electroreduction via engineering active phases of Pd nanoparticles. Nano Res. 10, 2181–2191 (2017).
Zhang, J., Wang, Y. & Li, Y. Not one, not two, but at least three: activity origin of copper single-atom catalysts toward CO2/CO electroreduction to C2+ products. J. Am. Chem. Soc. 146, 14954–14958 (2024).
Xie, G. et al. Dual-metal sites drive tandem electrocatalytic CO2 to C2+ products. Angew. Chem. Int. Ed. 63, e202412568 (2024).
Chen, Y. et al. Efficient multicarbon formation in acidic CO2 reduction via tandem electrocatalysis. Nat. Nanotech. 19, 311–318 (2024).
Zou, J., Lee, C. Y. & Wallace, G. G. Boosting formate production from CO2 at high current densities over a wide electrochemical potential window on a SnS catalyst. Adv. Sci. 8, 2004521 (2021).
Mani, F., Peruzzini, M. & Stoppioni, P. Combined process of CO2 capture by potassium carbonate and production of basic zinc (II) carbonates: CO2 release from bicarbonate solutions at room temperature and pressure. Energy Fuels 22, 1714–1719 (2008).
Xing, Z., Hu, L., Ripatti, D. S., Hu, X. & Feng, X. Enhancing carbon dioxide gas-diffusion electrolysis by creating a hydrophobic catalyst microenvironment. Nat. Commun. 12, 136 (2021).
Li, A. et al. Three-phase photocatalysis for the enhanced selectivity and activity of CO2 reduction on a hydrophobic surface. Angew. Chem. Int. Ed. 58, 14549–14555 (2019).
Monteiro, M. C. et al. Time-resolved local pH measurements during CO2 reduction using scanning electrochemical microscopy: buffering and tip effects. JACS Au 1, 1915–1924 (2021).
Li, L. et al. Hydrophobicity graded gas diffusion layer for stable electrochemical reduction of CO2. Angew. Chem. Int. Ed. 61, e202208534 (2022).
McKee, M. et al. Hydrophobic assembly of molecular catalysts at the gas–liquid–solid interface drives highly selective CO2 electromethanation. Nat. Chem. 17, 92–100 (2025).
Wu, Y. et al. Mitigating electrolyte flooding for electrochemical CO2 reduction via infiltration of hydrophobic particles in a gas diffusion layer. ACS Energy Lett. 7, 2884–2892 (2022).
Song, H. et al. Overcoming chemical and mechanical instabilities in lithium metal anodes with sustainable and eco-friendly artificial SEI layer. Adv. Mater. 36, 2407381 (2024).
Moorthy, M. et al. A series of hybrid multifunctional interfaces as artificial SEI layer for realizing dendrite free, and long-life sodium metal anodes. Adv. Funct. Mater. 33, 2300135 (2023).
Hu, T. et al. Impact of the local environment on Li ion transport in inorganic components of solid electrolyte interphases. J. Am. Chem. Soc. 145, 1327–1333 (2022).
Zhang, L. et al. Solvent-derived inorganic F and N-rich solid electrolyte interface for stable lithium metal batteries. Electrochim. Acta 503, 144909 (2024).
Samdani, J. S. et al. The identification of specific N-configuration responsible for Li-ion storage in N-doped porous carbon nanofibers: an ex-situ study. J. Power Sources 483, 229174 (2021).
Xue, H. et al. Aqueous formate-based Li–CO2 battery with low charge overpotential and high working voltage. Adv. Energy Mater. 11, 2101630 (2021).
Im, E., Ryu, J. H., Baek, K., Moon, G. D. & Kang, S. J. “Water-in-salt” and NASICON electrolyte-based Na–CO2 battery. Energy Storage Mater. 37, 424–432 (2021).
Kim, C. et al. Efficient CO2 utilization via a hybrid Na–CO2 system based on CO2 dissolution. iScience 9, 278–285 (2018).
Yang, X. et al. Upgrading cycling stability and capability of hybrid Na–CO2 batteries via tailoring reaction environment for efficient conversion CO2 to HCOOH. Adv. Energy Mater. 14, 2304365 (2024).
Li, J. et al. Li–CO2 batteries efficiently working at ultra-low temperatures. Adv. Funct. Mater. 30, 2001619 (2020).
Xue, T. et al. Quasi-solid-state electrolytes: bridging the gap between solid and liquid electrolytes for zinc-ion batteries. J. Chem. Eng. 514, 162994 (2025).
Na, D. et al. Li1.4Al0.4Ti1.6(PO4)3 inorganic solid electrolyte for all-solid-state Li–CO2 batteries with MWCNT and Ru nanoparticle catalysts. Mater. Today Energy 38, 101418 (2023).
Yoon, B. et al. Li1.4Al0.4Ge0.1Ti1.5(PO4)3: a stable solid electrolyte for Li–CO2 batteries. Mater. Chem. Phys. 322, 129583 (2024).
Lu, X. et al. Polymer-based solid-state electrolytes for high-energy-density lithium-ion batteries — review. Adv. Energy Mater. 13, 2301746 (2023).
Guan, D.-H. et al. All-solid-state photo-assisted Li–CO2 battery working at an ultra-wide operation temperature. ACS Nano 16, 12364–12376 (2022).
Li, Z. et al. Tailoring polymer electrolyte ionic conductivity for production of low-temperature operating quasi-all-solid-state lithium metal batteries. Nat. Commun. 14, 482 (2023).
Du, Y. et al. A rechargeable all-solid-state Li–CO2 battery using a Li1.5Al0.5Ge1.5(PO4)3 ceramic electrolyte and nanoscale RuO2 catalyst. J. Mater. Chem. A 9, 9581–9585 (2021).
Pasta, M., Wessells, C. D., Huggins, R. A. & Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat. Commun. 3, 1149 (2012).
Goyal, A., Marcandalli, G., Mints, V. A. & Koper, M. T. Competition between CO2 reduction and hydrogen evolution on a gold electrode under well-defined mass transport conditions. J. Am. Chem. Soc. 142, 4154–4161 (2020).
Sui, Y. & Ji, X. Anticatalytic strategies to suppress water electrolysis in aqueous batteries. Chem. Rev. 121, 6654–6695 (2021).
Wang, M. et al. Salting-out effect promoting highly efficient ambient ammonia synthesis. Nat. Commun. 12, 3198 (2021).
Singh, M. et al. Boosted capacity and stability of aqueous iron-sulfur battery using DMSO as an electrolyte additive. Energy Storage Mater. 75, 103965 (2025).
Liu, Y. et al. Integrated energy storage and CO2 conversion using an aqueous battery with tamed asymmetric reactions. Nat. Commun. 15, 977 (2024).
Wang, T. et al. Inhibiting anodic parasitic reaction for high-performance Al–air batteries via a multi-functional hybrid additive. J. Power Sources 579, 233294 (2023).
Wu, M. et al. Aqueous Zn-based rechargeable batteries: recent progress and future perspectives. InfoMat 4, e12265 (2022).
Gao, S. et al. Metal-free bifunctional ordered mesoporous carbon for reversible Zn–CO2 batteries. Small Methods 5, 2001039 (2021).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Ma, Z. et al. CO2 electroreduction to multicarbon products in strongly acidic electrolyte via synergistically modulating the local microenvironment. Nat. Commun. 13, 7596 (2022).
Chen, T. et al. Enabling solid-electrolyte interphase formation prior to water reduction in aqueous zinc batteries by mild protic chemistry. Angew. Chem. Int. Ed. 64, e202424642 (2025).
Yang, M. et al. Li2CO3/LiF-rich solid electrolyte interface stabilized lithium metal anodes for durable Li–CO2 batteries. Energy Storage Mater. 73, 103843 (2024).
Wu, W. et al. Addressing the carbonate issue: electrocatalysts for acidic CO2 reduction reaction. Adv. Mater. 37, 2312894 (2025).
Lu, J. et al. Synergistic effect of CO2 in accelerating the galvanic corrosion of lithium/sodium anodes in alkali metal–carbon dioxide batteries. ACS Nano 18, 10930–10945 (2024).
Qiu, F. et al. Towards a stable Li–CO2 battery: the effects of CO2 to the Li metal anode. Energy Storage Mater. 26, 443–447 (2020).
Hu, C. et al. High-performance, long-life, rechargeable Li–CO2 batteries based on a 3D holey graphene cathode implanted with single iron atoms. Adv. Mater. 32, 1907436 (2020).
Chen, C.-J. et al. Improvement of lithium anode deterioration for ameliorating cyclabilities of non-aqueous Li–CO2 batteries. Nanoscale 12, 8385–8396 (2020).
Li, J. et al. Strategies to anode protection in lithium metal battery: a review. InfoMat 3, 1333–1363 (2021).
Zhang, S. et al. Protecting Li-metal anode with ethylenediamine-based layer and in-situ formed gel polymer electrolyte to construct the high-performance Li–CO2 battery. J. Power Sources 506, 230226 (2021).
Qi, G. et al. Flexible Li–CO2 batteries with boosted reaction kinetics and cyclelife enabled by heterostructured Mo3N2@TiN cathode and interface-protected Li anode. Small 20, 2309064 (2024).
Song, Y. et al. An atmosphere-responsive protective strategy based on deciphering the anode degradation mechanism in Li–CO2 batteries. Angew. Chem. Int. Ed. 64, e202507865 (2025).
Cheng, X.-B., Zhang, R., Zhao, C.-Z. & Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 117, 10403–10473 (2017).
Ma, Y. et al. Electrochemically dealloyed 3D porous copper nanostructure as anode current collector of Li–metal batteries. Small 19, 2301731 (2023).
Lu, Y. et al. Rechargeable K–CO2 batteries with a KSn anode and a carboxyl-containing carbon nanotube cathode catalyst. Angew. Chem. Int. Ed. 60, 9540–9545 (2021).
Hu, X. et al. Quasi-solid state rechargeable Na-CO2 batteries with reduced graphene oxide Na anodes. Sci. Adv. 3, e1602396 (2017).
Khezri, R., Motlagh, S. R., Etesami, M., Mohamad, A. A. & Kheawhom, S. in Corrosion and Degradation in Fuel Cells, Supercapacitors and Batteries (ed. Saji, V. S.) 425–442 (Springer, 2024).
Guo, Y., Zhang, R., Zhang, S. & Zhi, C. Recent advances in Zn–CO2 batteries for the co-production of electricity and carbonaceous fuels. Nano Mater. Sci. https://doi.org/10.1016/j.nanoms.2022.09.004 (2022).
Wang, H. et al. Stabilization perspective on metal anodes for aqueous batteries. Adv. Energy Mater. 11, 2000962 (2021).
Zheng, J. et al. Spontaneous and field-induced crystallographic reorientation of metal electrodeposits at battery anodes. Sci. Adv. 6, eabb1122 (2020).
Liu, J. et al. A Cu–Ag double-layer coating strategy for stable and reversible Zn metal anodes. J. Colloid Interface Sci. 665, 163–171 (2024).
Zhang, Q. et al. Revealing the role of crystal orientation of protective layers for stable zinc anode. Nat. Commun. 11, 3961 (2020).
Wang, K., Pickering, H. & Weil, K. Corrosion inhibition of zinc by benzotriazole with an electrochemical quartz crystal microbalance. J. Electrochem. Soc. 150, B176 (2003).
Liang, Y. & Yao, Y. Designing modern aqueous batteries. Nat. Rev. Mater. 8, 109–122 (2023).
Li, L. et al. Gas concentration-driven LiOH chemistry in Li–CO2 batteries. Electrochem. Commun. 160, 107669 (2024).
Hu, Y. et al. Suppressing local dendrite hotspots via current density redistribution using a superlithiophilic membrane for stable lithium metal anode. Adv. Sci. 10, 2206995 (2023).
Nemani, V. P. & Smith, K. C. Analysis of crossover-induced capacity fade in redox flow batteries with non-selective separators. J. Electrochem. Soc. 165, A3144 (2018).
Yin, W., Grimaud, A., Azcarate, I., Yang, C. & Tarascon, J.-M. Electrochemical reduction of CO2 mediated by quinone derivatives: implication for Li–CO2 battery. J. Phys. Chem. C 122, 6546–6554 (2018).
Zhang, X., Zhu, J. & Sahraei, E. Degradation of battery separators under charge-discharge cycles. RSC Adv. 7, 56099–56107 (2017).
Gogia, A. et al. Binder-free, thin-film ceramic-coated separators for improved safety of lithium-ion batteries. ACS Omega 6, 4204–4211 (2021).
Li, H. et al. Engineering and construction of multi-functional Janus separator for high-stability Li–CO2 battery. J. Colloid Interface Sci. 683, 335–346 (2025).
Liu, Y. et al. Anion-repulsive polyoxometalate@MOF-modified separators for dendrite-free and high-rate lithium batteries. Interdisc. Mater. 4, 190–200 (2025).
Li, M. et al. Tin asymmetric membranes for high capacity sodium ion battery anodes. Mater. Today Commun. 24, 100998 (2020).
Wu, S., Li, G., Liu, H. & Duan, H. Porous polymer membrane with uniform lithium-ion transport via phase separation for stable lithium metal batteries. J. Power Sources 547, 232018 (2022).
Klose, C. et al. All-hydrocarbon MEA for PEM water electrolysis combining low hydrogen crossover and high efficiency. Adv. Energy Mater. 10, 1903995 (2020).
Tschinder, T., Schaffer, T., Fraser, S. & Hacker, V. Electro-osmotic drag of methanol in proton exchange membranes. J. Appl. Electrochem. 37, 711–716 (2007).
Habibzadeh, F. et al. Ion exchange membranes in electrochemical CO2 reduction processes. Electrochem. Energy Rev. 6, 26 (2023).
Yan, Z., Hitt, J. L., Zeng, Z., Hickner, M. A. & Mallouk, T. E. Improving the efficiency of CO2 electrolysis by using a bipolar membrane with a weak-acid cation exchange layer. Nat. Chem. 13, 33–40 (2021).
Willdorf-Cohen, S. et al. Alkaline stability of anion-exchange membranes. ACS Appl. Energy Mater. 6, 1085–1092 (2023).
Riemer, M., Duval-Dachary, S. & Bachmann, T. M. Environmental implications of reducing the platinum group metal loading in fuel cells and electrolysers: anion exchange membrane versus proton exchange membrane cells. Sustain. Energy Technol. Assess. 56, 103086 (2023).
Giesbrecht, P. K. & Freund, M. S. Influence of the pH gradient on bipolar membrane operation. ACS Electrochem. 1, 667–677 (2024).
Tufa, R. A. et al. Bipolar membrane and interface materials for electrochemical energy systems. ACS Appl. Energy Mater. 4, 7419–7439 (2021).
Strathmann, H., Krol, J., Rapp, H.-J. & Eigenberger, G. Limiting current density and water dissociation in bipolar membranes. J. Membr. Sci. 125, 123–142 (1997).
Li, Y. et al. Strong electronic interactions of the abundant Cu/Ce interfaces stabilized Cu2O for efficient CO2 electroreduction to C2+ products under large current density. Adv. Funct. Mater. https://doi.org/10.1002/adfm.202509899 (2025).
Sharon, D. et al. Oxidation of dimethyl sulfoxide solutions by electrochemical reduction of oxygen. J. Phys. Chem. Lett. 4, 3115–3119 (2013).
Zhang, P.-F. et al. Li–CO2/O2 battery operating at ultra-low overpotential and low O2 content on Pt/CNT catalyst. J. Chem. Eng. 448, 137541 (2022).
Liu, L.-Y. et al. Boosting oxygen-resistant CO2 electroreduction reaction in acidic media over conjugated frameworks. J. Mater. Chem. A 12, 9486–9493 (2024).
Liu, W. et al. The impact of a trace amount of water in an electrolyte on the performance of Li-ion batteries — an empirical kinetic model approach. Int. J. Energy Res. 46, 7988–7995 (2022).
Zhou, H. et al. The influence of water in electrodes on the solid electrolyte interphase film of micro lithium-ion batteries for the wireless headphone. J. Colloid Interface Sci. 606, 1729–1736 (2022).
Jeanmonod, G., Diethelm, S. & Van Herle, J. The effect of SO2 on the Ni–YSZ electrode of a solid oxide electrolyzer cell operated in co-electrolysis. J. Phys. Energy 2, 034002 (2020).
Hernik, B., Brudziana, P., Klon, R. & Pronobis, M. Numerical studies of the influence of flue gas recirculation into primary air on NOx formation, CO emission, and low-NOx waterwall corrosion in the OP 650 boiler. Energies 17, 2227 (2024).
Khurram, A., He, M. & Gallant, B. M. Tailoring the discharge reaction in Li–CO2 batteries through incorporation of CO2 capture chemistry. Joule 2, 2649–2666 (2018).
Li, M., Yang, K., Abdinejad, M., Zhao, C. & Burdyny, T. Advancing integrated CO2 electrochemical conversion with amine-based CO2 capture: a review. Nanoscale 14, 11892–11908 (2022).
Franz, H. B. et al. Initial SAM calibration gas experiments on Mars: quadrupole mass spectrometer results and implications. Planet. Space Sci. 138, 44–54 (2017).
Yang, M., Chen, L., Li, H. & Wu, F. Air/water stability problems and solutions for lithium batteries. Energy Mater. Adv. 2022, 9842651 (2022).
Wang, K., Wu, Y., Cao, X., Gu, L. & Hu, J. A Zn–CO2 flow battery generating electricity and methane. Adv. Funct. Mater. 30, 1908965 (2020).
Sedighi, M., Usefi, M. M. B., Ismail, A. F. & Ghasemi, M. Environmental sustainability and ions removal through electrodialysis desalination: operating conditions and process parameters. Desalination 549, 116319 (2023).
Abanades, J. C., Criado, Y. A. & White, H. I. Direct capture of carbon dioxide from the atmosphere using bricks of calcium hydroxide. Cell Rep. Phys. Sci. 4, 101339 (2023).
Kumai, K., Miyashiro, H., Kobayashi, Y., Takei, K. & Ishikawa, R. Gas generation mechanism due to electrolyte decomposition in commercial lithium-ion cell. J. Power Sources 81, 715–719 (1999).
Xue, Z., Qin, L., Jiang, J., Mu, T. & Gao, G. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 20, 8382–8402 (2018).
Wang, X. et al. Investigation on the combustion and explosion characteristics of lithium iron phosphate battery thermal runaway gas: effects of initial pressure and ignition energy. Fuel 399, 135605 (2025).
Chen, X., Granda-Marulanda, L. P., McCrum, I. T. & Koper, M. T. How palladium inhibits CO poisoning during electrocatalytic formic acid oxidation and carbon dioxide reduction. Nat. Commun. 13, 38 (2022).
Lee, S.-M. et al. Improvement in self-discharge of Zn anode by applying surface modification for Zn–air batteries with high energy density. J. Power Sources 227, 177–184 (2013).
Sun, J., Ling, Y., Zhang, Q., Yu, X. & Yang, Z. Simultaneous enhancements in stability and CO tolerance of Pt electrocatalyst by double poly (vinyl pyrrolidone) coatings. RSC Adv. 7, 29839–29843 (2017).
Yang, Z., Berber, M. R. & Nakashima, N. A polymer-coated carbon black-based fuel cell electrocatalyst with high CO-tolerance and durability in direct methanol oxidation. J. Mater. Chem. A 2, 18875–18880 (2014).
Yang, Q. et al. Efficient and stable PtFe alloy catalyst for electrocatalytic methanol oxidation with high resistance to CO. J. Energy Chem. 90, 327–336 (2024).
Yang, L. et al. Energy-efficient separation alternatives: metal–organic frameworks and membranes for hydrocarbon separation. Chem. Soc. Rev. 49, 5359–5406 (2020).
Feng, X. et al. A coupled electrochemical-thermal failure model for predicting the thermal runaway behavior of lithium-ion batteries. J. Electrochem. Soc. 165, A3748–A3765 (2018).
Song, L., Zheng, Y., Xiao, Z., Wang, C. & Long, T. Review on thermal runaway of lithium-ion batteries for electric vehicles. J. Electron. Mater. 51, 30–46 (2022).
Chavan, S. et al. Thermal runaway and mitigation strategies for electric vehicle lithium-ion batteries using battery cooling approach: a review of the current status and challenges. J. Energy Storage 72, 108569 (2023).
Luo, P., Gao, K., Hu, L., Chen, B. & Zhang, Y. Adaptive hybrid cooling strategy to mitigate battery thermal runaway considering natural convection in phase change material. Appl. Energy 361, 122920 (2024).
Richard, M. & Dahn, J. Accelerating rate calorimetry study on the thermal stability of lithium intercalated graphite in electrolyte. I. Experimental. J. Electrochem. Soc. 146, 2068 (1999).
Li, Y. et al. Early warning method for thermal runaway of lithium-ion batteries under thermal abuse condition based on online electrochemical impedance monitoring. J. Energy Chem. 92, 74–86 (2024).
Kim, S. W., Kwak, E., Kim, J.-H., Oh, K.-Y. & Lee, S. Modeling and prediction of lithium-ion battery thermal runaway via multiphysics-informed neural network. J. Energy Storage 60, 106654 (2023).
Wang, Y. et al. Investigation on calendar experiment and failure mechanism of lithium-ion battery electrolyte leakage. J. Energy Storage 54, 105286 (2022).
Liu, Y. K. et al. Research progresses of liquid electrolytes in lithium-ion batteries. Small 19, 2205315 (2023).
Chang, Z. et al. A stable high-voltage lithium-ion battery realized by an in-built water scavenger. Energy Environ. Sci. 13, 1197–1204 (2020).
Miao, K., Qin, J., Yang, J. & Kang, X. Synergy of Ni nanoclusters and single atom site: size effect on the performance of electrochemical CO2 reduction reaction and rechargeable Zn–CO2 batteries. Adv. Funct. Mater. 34, 2316824 (2024).
Liu, X. et al. Mapping the design of electrolyte materials for electrically rechargeable zinc–air batteries. Adv. Mater. 33, 2006461 (2021).
Xie, J., Zhou, Z. & Wang, Y. Metal–CO2 batteries at the crossroad to practical energy storage and CO2 recycle. Adv. Funct. Mater. 30, 1908285 (2020).
Innocenti, A., Bresser, D., Garche, J. & Passerini, S. A critical discussion of the current availability of lithium and zinc for use in batteries. Nat. Commun. 15, 4068 (2024).
Sun, K. E. et al. Suppression of dendrite formation and corrosion on zinc anode of secondary aqueous batteries. ACS Appl. Mater. Interfaces 9, 9681–9687 (2017).
Hu, X., An, A. K. & Chopra, S. S. Life cycle assessment of the polyvinylidene fluoride polymer with applications in various emerging technologies. ACS Sustain. Chem. Eng. 10, 5708–5718 (2022).
Greitzer, M. in Powerfuels (eds Bullerdiek, N. et al.) 257–279 (Springer, 2025).
Morris, M. I. R. & Hicks, A. Life cycle assessment of stainless-steel reusable speculums versus disposable acrylic speculums in a university clinic setting: a case study. Environ. Res. Commun. 4, 025002 (2022).
Wang, J. et al. Green recycling of spent Li-ion battery cathodes via deep-eutectic solvents. Energy Environ. Sci. 17, 867884 (2024).
Lu, B. et al. High-efficiency leaching of valuable metals from waste Li-ion batteries using deep eutectic solvents. Environ. Res. 212, 113286 (2022).
Norgate, T. E., Jahanshahi, S. & Rankin, W. J. Assessing the environmental impact of metal production processes. J. Clean. Prod. 15, 838–848 (2007).
Sharma, R., Gyergyek, S., Lund, P. B. & Andersen, S. M. Recovery of Pt and Ru from spent low-temperature polymer electrolyte membrane fuel cell electrodes and recycling of Pt by direct redeposition of the dissolved precursor on carbon. ACS Appl. Energy Mater. 4, 6842–6852 (2021).
Otgonbayar, U., Sandig-Predzymirska, L., Thiere, A. & Charitos, A. Solvent extraction of Pt, Ru, and Ir using Cyanex 923 in chloride media to develop a recycling route for spent polymer electrolyte membrane (PEM) electrolyzers. Hydrometallurgy 226, 106303 (2024).
Li, X. et al. Bamboo-like nitrogen-doped carbon nanotube forests as durable metal-free catalysts for self-powered flexible Li–CO2 batteries. Adv. Mater. 31, 1903852 (2019).
Joo, S.-H., Shin, D., Oh, C., Wang, J.-P. & Shin, S. M. Extraction of manganese by alkyl monocarboxylic acid in a mixed extractant from a leaching solution of spent lithium-ion battery ternary cathodic material. J. Power Sources 305, 175–181 (2016).
Rothermel, S. et al. Graphite recycling from spent lithium-ion batteries. ChemSusChem 9, 3473–3484 (2016).
He, K., Zhang, Z.-Y., Alai, L. & Zhang, F.-S. A green process for exfoliating electrode materials and simultaneously extracting electrolyte from spent lithium-ion batteries. J. Hazard. Mater. 375, 43–51 (2019).
Wang, P. et al. Integrated system for electrolyte recovery, product separation, and CO2 capture in CO2 reduction. Nat. Commun. 16, 731 (2025).
Kołodziejczak-Radzimska, A., Jesionowski, T. & Krysztafkiewicz, A. Obtaining zinc oxide from aqueous solutions of KOH and Zn(CH3COO)2. Physicochem. Probl. Miner. Process. 44, 93–102 (2010).
Lu, B. et al. Recycled tandem catalysts promising ultralow overpotential Li–CO2 batteries. Adv. Mater. 36, 2309264 (2024).
Wang, Y., Lv, H., Sun, L. & Liu, B. Mesoporous noble metal-metalloid/nonmetal alloy nanomaterials: designing highly efficient catalysts. ACS Nano 15, 18661–18670 (2021).
Wu, T., Sun, M.-Z. & Huang, B.-L. Non-noble metal-based bifunctional electrocatalysts for hydrogen production. Rare Met. 41, 2169–2183 (2022).
Fardood, S. T. et al. Green synthesis of ZnO nanoparticles via sol–gel method and investigation of its application in solvent-free synthesis of 12-aryl-tetrahydrobenzo [α] xanthene-11-one derivatives under microwave irradiation. Chem. Methodol. 3, 632–642 (2019).
Huang, H. et al. Polyanionic cathode materials: a comparison between Na-ion and K-ion batteries. Adv. Energy Mater. 14, 2304251 (2024).
Ziegler, M. S. & Trancik, J. E. Re-examining rates of lithium-ion battery technology improvement and cost decline. Energy Environ. Sci. 14, 1635–1651 (2021).
Deckenbach, D. & Schneider, J. J. A long-overlooked pitfall in rechargeable zinc-air batteries: proper electrode balancing. Adv. Mater. Interfaces 10, 2202494 (2023).
Chen, P. et al. Recent progress in electrolytes for Zn–air batteries. Front. Chem. 8, 372 (2020).
Xia, Q. et al. Integration of CO2 capture and electrochemical conversion: focus review. ACS Energy Lett. 8, 2840–2857 (2023).
Sullivan, I. et al. Coupling electrochemical CO2 conversion with CO2 capture. Nat. Catal. 4, 952–958 (2021).
Shen, C., Wycisk, R. & Pintauro, P. N. High performance electrospun bipolar membrane with a 3D junction. Energy Environ. Sci. 10, 1435–1442 (2017).
Oener, S. Z., Foster, M. J. & Boettcher, S. W. Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science 369, 1099–1103 (2020).
Ge, Z. et al. Beneficial use of a coordination complex as the junction catalyst in a bipolar membrane. ACS Appl. Energy Mater. 3, 5765–5773 (2020).
Zeng, M. et al. Reaction environment regulation for electrocatalytic CO2 reduction in acids. Angew. Chem. Int. Ed. 63, e202404574 (2024).
Acknowledgements
The authors thank the Australian Research Council (ARC) for support (grants CE230100017, FL210100050, DP210101486, DE240100159, DE250101071 and DP250102252). J.Z. acknowledges support from Sandland Bequest_Zou and an AINSE Early Career Researcher Grant (ECRG).
Author information
Authors and Affiliations
Contributions
J.Z., D.G. and J.A.Y. researched data for the article. All authors contributed substantially to discussion of the content. J.A.Y. performed the life cycle assessment and techno-economic analyses. J.Z. and D.G. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clean Technology thanks Chandra S. Sharma, who co-reviewed with Ankit Chourasia; Shuya Wei; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Cathode–electrolyte interface
-
Both Faradaic and non-Faradaic processes occur at the cathode–electrolyte interface. When the cathode and electrolyte come into ohmic contact, charge-transfer processes (oxidation and reduction) occur (Faradaic). Changes due to adsorption and desorption result in the flow of transient external currents (non-Faradaic) at the interface.
- Dendrite growth
-
Dendrites are tiny, branch-like crystal structures extending from metal anodes because of metal diffusion and plating. Dendrites can penetrate the separator, causing a short circuit in the battery.
- Faradaic efficiency
-
A metric for product selectivity in electrochemical reactions, defined as the fraction of electrical charge consumed during the reaction for selective product generation (Qprod) divided by the total charge (Qtotal) passed, expressed in per cent.
- Gutmann number
-
The Gutmann donor number describes electron-donating and H-bond-acceptor ability. It helps to determine the hydrogen-bonding environment and influences the rate of the hydrogen evolution reaction (HER) in aqueous electrolytes.
- Linear scaling relationships
-
Theoretical tools providing constraints in catalyst design by relating the binding energy of catalyst intermediates and adsorbates across a wide range of catalysts.
- Rocking chair
-
The rocking chair battery mechanism involves shuttling of metal ions between the cathode and anode.
- Sabatier principle
-
The Sabatier principle states that optimal catalytic activity occurs when the interaction between a catalyst and a reactant is neither too strong nor too weak to facilitate both adsorption and desorption.
- Solid electrolyte interphase
-
(SEI). A layer formed at the electrode–electrolyte interface that passivates the electrode surface. It should be stable during battery cycling and accelerate metal-ion transfer.
- Spillover effects
-
Spillover energy determines the stability of intermediates at the dopant or host metal sites, which induce the spillover effect in single-atom alloys. The spillover effect in single-atom alloys involves the migration of adsorbed species from a secondary single-atom active site (dopant) towards the primary single atom.
- Synergistic optimization
-
Refers to the introduction of multiple active sites in a catalyst to improve the reaction kinetics, in which the combined catalytic activity of different active sites is greater compared to their individual activities thanks to cooperative interactions.
- Tandem reactions
-
A tandem reaction in electrochemical CO2 reduction is a sequential process in which CO2 is first converted into intermediates such as CO and then further reduced to final products (C2+ products).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, J., Gupta, D., Yuwono, J.A. et al. The promises and reality of metal–CO2 batteries. Nat. Rev. Clean Technol. (2025). https://doi.org/10.1038/s44359-025-00099-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s44359-025-00099-1