Abstract
Study design:
Previous studies have shown that transplantation of bone marrow stromal cells (MSCs) in animal models of spinal cord injury (SCI) encourages functional recovery. Here, we have examined the growth in cell culture of MSCs isolated from individuals with SCI, compared with non-SCI donors.
Setting:
Centre for Spinal Studies, Midland Centre for Spinal Injuries, RJAH Orthopaedic Hospital, Oswestry, UK.
Methods:
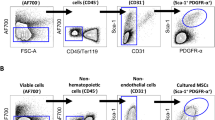
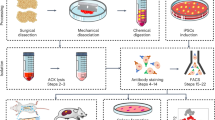
Bone marrow was harvested from the iliac crest of donors with long-term SCI (>3 months, n=9) or from non-SCI donors (n=7). Mononuclear cells were plated out into tissue culture flasks and the adherent MSC population subsequently expanded in monolayer culture. MSC were passaged by trypsinization at 70% confluence and routinely seeded into new flasks at a density of 5 × 103 cells per cm2. Expanded cell cultures were phenotypically characterized by CD-immunoprofiling and by their differentiation potential along chondrocyte, osteoblast and adipocyte lineages. The influence of cell-seeding density on the rate of cell culture expansion and degree of cell senescence was examined in separate experiments.
Results:
In SCI, but not in non-SCI donors the number of adherent cells harvested at passage I was age-related. The proliferation rate (culture doubling times) between passages I and II was significantly greater in cultures from SCI donors with cervical lesions than in those with thoracic lesions. There was no significant difference, however, in either the overall cell harvests at passages I or II or in the culture doubling times between SCI and non-SCI donors. At passage II, more than 95% of cells were CD34−ve, CD45−ve and CD105+ve, which is characteristic of human MSC cultures. Furthermore, passage II cells differentiated along all three mesenchymal lineages tested. Seeding passage I–III cells at cell densities lower than 5 × 103 cells per cm2 significantly reduced culture doubling times and significantly increased overall cell harvests while having no effect on cell senescence.
Conclusion:
MSCs from individuals with SCI can be successfully isolated and expanded in culture; this is encouraging for the future development of MSC transplantation therapies to treat SCI. Age, level of spinal injury and cell-seeding density were all found to relate to the growth kinetics of MSC cultures in vitro, albeit in a small sample group. Therefore, these factors should be considered if either the overall number or the timing of MSC transplantations post-injury is found to relate to functional recovery.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglar R, Mosca JD et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147.
Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA 2002; 99: 2199–2204.
Ankeny DP, McTigue DM, Jakeman LB . Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp Neurol 2004; 190: 17–31.
Neuhuber B, Himes BT, Shumsky JS, Gallo G, Fischer I . Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res 2005; 1035: 73–85.
Himes BT, Neuhuber B, Coleman C, Swanger R, Kopen GC, Wanger J et al. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair 2006; 20: 278–296.
Zurita M, Vaquero J . Functional recovery in chronic paraplegia after bone marrow cells transplantation. Neuroreport 2004; 15: 1105–1108.
Zurita M, Vaquero J . Bone marrow stromal cells can achieve cure of chronic paraplegia in rats: functional and morphological outcome one year after transplantation. Neurosci Lett 2006; 402: 51–56.
Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG . Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol 2006; 198: 54–64.
Dezawa M, Hoshino M, Nabozny GH, Ide C . Marrow stromal cells: implications in health and disease of the nervous system. Curr Mol Med 2005; 5: 723–732.
Wright KT, El Masri W, Osman A, Roberts S, Chamberlain G, Ashton BA et al. Bone marrow stromal cells stimulate neurite outgrowth over neural proteoglycans (CSPG), myelin associated glycoprotein and Nogo-A. Biochem Biophys Res Commun 2007; 354: 559–566.
Roberts S, Evans HE, Kletsas D, Jaffray DC, Eisenstein SM . Senescence in human intervertebral discs. Eur Spine J 2006; 15 (Suppl 3): 312–316.
Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE et al. A small proportion of mesenchymal stem cells strongly express functionally active CXCR4 receptor capable of promoting migration of bone marrow. Blood 2004; 104: 2643–2645.
Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP . Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem 1997; 64: 295–312.
Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU . In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 1998; 238: 265–272.
Iversen PO, Hjeltner N, Holm B, Flatebo T, Strom-Gundersen I, Ronning W et al. Depressed immunity and impaired proliferation of haematopoietic progenitor cells in patients with complete spinal cord injury. Blood 2000; 96: 2081–2083.
Kliesch WF, Cruse JM, Lewis RE, Bishop GR, Brackin B, Lampton JA et al. Restoration of depressed immune function in spinal cord injury patients receiving rehabilitation therapy. Paraplegia 1996; 34: 82–90.
Chernykh ER, Shevela EY, Leplina OY, Tikhonova MA, Ostanin AA, Kulagin AD et al. Characteristics of bone marrow cells under conditions of impaired innervation in patients with spinal trauma. Bull Exp Biol Med 2006; 141: 117–120.
Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem 2006; 97: 744–754.
Yamaguchi A, Komori T, Suda T . Regulation of osteoblastic differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocrine Rev 2000; 21: 393–411.
Gos M, Miloszewska J, Swoboda P, Trembacz H, Skierski J, Janik P et al. Cellular quiescence induced by contact inhibition or serum withdrawal in C3H10T1/2 cells. Cell Prolif 2005; 38: 107–116.
Acknowledgements
For assistance in the collection of bone marrow aspirates, we would like to thank Dr Anthony Powell of the Haematology Department, Royal Shrewsbury Hospital, Shropshire, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wright, K., Masri, W., Osman, A. et al. The cell culture expansion of bone marrow stromal cells from humans with spinal cord injury: implications for future cell transplantation therapy. Spinal Cord 46, 811–817 (2008). https://doi.org/10.1038/sc.2008.77
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sc.2008.77
Keywords
This article is cited by
-
Spinal cord injury causes chronic bone marrow failure
Nature Communications (2020)
-
Artificial collagen-filament scaffold promotes axon regeneration and long tract reconstruction in a rat model of spinal cord transection
Medical Molecular Morphology (2015)
-
Viability, growth kinetics and stem cell markers of single and clustered cells in human intervertebral discs: implications for regenerative therapies
European Spine Journal (2014)
-
Cell sources for nucleus pulposus regeneration
European Spine Journal (2014)
-
Neural stem cells for spinal cord repair
Cell and Tissue Research (2012)