Abstract
Study design:
Descriptive report.
Objectives:
To describe screening to recruitment (S:R) ratios and discuss their use for planning and implementing research among individuals with spinal cord injury (SCI).
Setting:
Toronto, Ontario, Canada.
Methods:
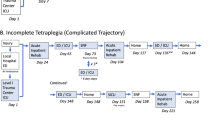
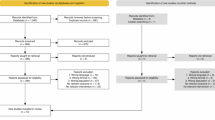
We calculated S:R ratios for SCI research by study methodology and nature of the exposure/intervention for 25 studies previously conducted in a tertiary SCI rehabilitation facility. Study methodologies included ten randomized controlled trials (RCTs), nine cohort studies and six panel studies. Exposures included seven rehabilitation interventions, and three drug studies, ten telephone interviews/chart abstractions (TI/CA) and five surveys. A S:R ratio was calculated for each study methodology, and exposure type, by dividing the number of consenting individuals who underwent screening by the number of eligible recruited participants enrolled in the study.
Results:
In terms of design, RCTs had the highest median S:R ratio (3:1), followed by cohort studies (2:1) and panel studies (2:1). In terms of intervention type, drug studies had the largest median S:R ratio (5:1), followed in descending order by rehabilitation studies (2:1), TI/CAs studies (2:1) and surveys (2:1).
Conclusions:
Reported S:R ratios varied substantially with study methodology and the associated study intervention exposure. Awareness of S:R ratios may assist researchers in estimating recruitment timelines, personnel needs and study budgets for a required sample size based on the planned study methodology and intended study exposure. We advocate for the routine reporting of S:R ratios to inform the success of future SCI research.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Farry A, Baxter D The incidence and prevalence of spinal cord injury in Canada. Overview and estimates based on current evidence. Rick Hansen Institute and Urban Futures: Vancouver, BC. 2010. Available from. http://fecst.inesss.qc.ca/fileadmin/documents/photos/LincidenceetlaprevalencedestraumamedullaireauCanada.pdf (accessed 29 October 2013)..
Ontario Neurotrauma Foundation Spinal cord injury. Available from. http://onf.org/ (accessed 9 May 2013)..
Couris CM, Guilcher SJ, Munce SE, Fung K, Craven BC, Verrier M et al. Characteristics of adults with incident traumatic spinal cord injury in Ontario, Canada. Spinal Cord 2010; 48: 45–50.
National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. Birmingham, AL, USA. 2012.
U.S. Government Printing Office United States Public Law 107-280.. Rare Diseases Act of 2002. U.S. GPO: Washington, DC, USA. 2002.
The Council of the European Union. Council recommendation of 8 June 2009 on an action in the field of rare diseases. Off J Eur Union 2009; 151: 7–10.
Cardenas D, Yilmaz B . Recruitment of spinal cord injury patients to clinical trials: challenges and solutions. Top Spinal Cord Inj Rehabil 2006; 11: 12–23.
Campagnolo D, Kirshblum S . Spinal Cord Medicine 2nd edn, Lippincott Williams & Wilkins: Philadelphia, PA, USA. 2011.
Blanton S, Morris DM, Rettyman MG, McCulloch K, Redmond S, Light KE et al. Lessons learned in participant recruitment and retention: the EXCITE trial. Phys Ther 2006; 86: 1520–1533.
Bell KR, Hammond F, Hart T, Bickett AK, Temkin NR, Dikmen S . Participant recruitment and retention in rehabilitation research. Am J Phys Med Rehabil 2008; 87: 330–338.
Scales DC, Rubenfeld GD . Estimating sample size in critical care clinical trials. J Crit Care 2005; 20: 6–11.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12.
Aitken L, Gallagher R, Madronio C . Principles of recruitment and retention in clinical trials. Int J Nurs Pract 2003; 9: 338–346.
Saldaña SN, Hooper DK, Froehlich TE, Campbell KM, Prows CA, Sadhasivam S et al. Characteristics of successful recruitment in prospective pediatric pharmacogenetic studies. Clin Ther 2011; 33: 2072–2081.
Kaur G, Hutchison I, Mehanna H, Williamson P, Shaw R, Tudur Smith C . Barriers to recruitment for surgical trials in head and neck oncology: a survey of trial investigators. BMJ Open 2013; 3: 4.
Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement updated guidelines for reporting parallel group randomized trials. BMJ 2010; 340: 698–702.
Jones LAT, Lammertse DP, Charlifue SB, Kirshblum SC, Apple DF, Ragnarsson KT et al. A phase 2 autologous cellular therapy trial in patients with acute, complete spinal cord injury: pragmatics, recruitment, and demographics. Spinal Cord 2010; 48: 798–807.
Hemminki E, Hovi SL, Veerus P, Sevon T, Tuimala R, Rahu M et al. Blinding decreased recruitment in a prevention trial of postmenopausal hormone therapy. J Clin Epidemiol 2004; 57: 1237–1243.
Verrier M, Carson JR, Brisbois L, Craven BC Central Recruitment Project: Exploring feasibility and scalability for research studies in spinal cord injury. Poster presented at: Translating Neural Engineering and Novel Therapies, 5th National Spinal Cord Injury Conference; 2012 October 19–20; Toronto, Ontario, Canada.
Craven BC, Brisbois LM, Verrier MC Exploring the feasibility of central recruitment for subacute SCI patients. Poster presented at: Toronto Rehabilitation Institute Annual Research Day Conference; 2012 November 23; Toronto, Ontario, Canada.
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandernbroucke JP . STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007; 18: 800–804.
Leveille SG, Huang A, Tsai SB, Weingart SN, Iezzoni LI . Screening for chronic conditions using a patient internet portal: recruitment for an internet-based primary care intervention. J Gen Intern Med 2008; 23: 472–475.
Streiner DL, Norman GR . PDQ Epidemiology end edn. BC Decker, Inc: Hamilton, London. 1998.
Streiner DL, Norman GR . PDQ Epidemiology end edn BC Decker, Inc: Hamilton, London. 1998 pp 68.
Sibbald B, Roland M . Understanding controlled trials: why are randomized controlled trials important? BMJ 1998; 316: 201.
Fletcher RW, Fletcher SW . Clinical Epidemiology: The essentials. Lippincott Williams & Wilkins: Philadelphia, PA, USA. 2005.
Oleckno WA . Epidemiology. Concepts and Methods. Waveland Press, Inc: Long Grove, IL, USA. 2008.
Acknowledgements
This study was supported by the Ontario Neurotrauma Foundation (ONF Central Recruitment Grant #2011-SCI-Mentor-884). We acknowledge the support of the Toronto Rehabilitation Institute, which received funding under the Provincial Rehabilitation Research Program from the Ministry of Health and Long-Term Care in Ontario during the conduct of many of the studies whose data are presented. The views expressed do not necessarily reflect those of the Ministry. We wish to thank the participants in all included studies. Due to site-specific reporting, sample sizes may differ to those obtained from the full study results. L. Giangregorio received an Early Researcher Award from the Ministry of Research and Innovation, and a Canadian Institutes of Health Research New Investigator Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Craven, B., Balioussis, C., Hitzig, S. et al. Use of screening to recruitment ratios as a tool for planning and implementing spinal cord injury rehabilitation research. Spinal Cord 52, 764–768 (2014). https://doi.org/10.1038/sc.2014.126
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sc.2014.126