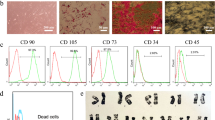
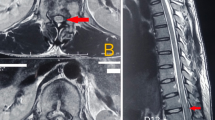
Abstract
Study design:
This is a clinical trial (phase 1).
Objectives:
The objective of this study was to asses the safety and feasibility of bone marrow mesenchymal stem cell (MSC) and Schwann cell (SC) co-injection through cerebral spinal fluid (CSF) for the treatment of patients with chronic spinal cord injury.
Methods:
Six subjects with complete spinal cord injury due to trauma according to International Standard of Neurological Classification for Spinal Cord Injury (ISNCSCI) developed by the American Spinal Injury Association were enrolled. They received autologous co-transplantation of MSC and SC through lumbar puncture. Neurological status of the patients was determined by ISNCSCI, as well as by assessment of functional status by Spinal Cord Independent Measure. Before and after cell transplantation, magnetic resonance imaging (MRI) was performed for all the patients. Before the procedure, all the patients underwent electromyography, urodynamic study (UDS) and MRI tractograghy. After transplantation, these assessments were performed in special cases when the patients reported any changes in motor function or any changes in urinary sensation.
Results:
Over the mean 30 months of follow-up, the radiological findings were unchanged without any evidence of neoplastic tissue overgrowth. American Spinal Injury Association class in one patient was changed from A to B, in addition to the improvement in indexes of UDS, especially bladder compliance, which was congruous with axonal regeneration detected in MRI tractography. No motor score improvement was observed among the patients.
Conclusion:
No adverse findings were detected at a mean of 30 months after autologous transplantation of the combination of MSCs and SCs through CSF. It may suggest the safety of this combination of cells for spinal cord regeneration.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fujiwara Y, Tanaka N, Ishida O, Fujimoto Y, Murakami T, Kajihara H et al. Intravenously injected neural progenitor cells of transgenic rats can migrate to the injured spinal cord and differentiate into neurons, astrocytes and oligodendrocytes. Neurosci Lett 2004; 366: 287–291.
Neirinckx V, Cantinieaux D, Coste C, Rogister B, Franzen R, Wislet-Gendebien S . Spinal cord injuries - how could adult mesenchymal and neural crest stem cells take up the challenge? Stem Cells 2013; 32: 829–843.
Oudega M, Xu X-M . Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma 2006; 23: 453–467.
Tator CH . Review of treatment trials in humanspinal cord injury: Issues, difficulties, and recommendations. Neurosurgery 2006; 59: 957–987.
Yazdani SO, Hafizi M, Zali A-R, Atashi A, Ashrafi F, Seddighi A-S et al. Safety and possible outcome assessment of autologous Schwann cell and bone marrow mesenchymal stromal cell co-transplantation for treatment of patients with chronic spinal cord injury. Cytotherapy 2013; 15: 782–791.
Satake K, Lou J, Lenke LG . Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine 2004; 29: 1971–1979.
Bakshi A, Barshinger AL, Swanger SA, Madhvani V, Shumsky JS, Neuhuber B et al. Lumbar puncture delivery of bone marrow stromal cells in spinal cord contusion: a novel method for minimally invasive cell transplantation. J Neurotrauma 2006; 23: 55–65.
Callera F, de Melo CM . Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells' migration into the injured site. Stem Cells Dev 2007; 16: 461–466.
Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, Laramee M et al. A multicenter international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord 2007; 45: 275–291.
Vroemen M, Aigner L, Winkler J, Weidner N . Adult neural progenitor cell grafts survive after acute spinal cord injury and integrate along axonal pathways. Eur J Neurosci 2003; 18: 743–751.
Ichihara K, Taguchi T, Shimada Y, Sakuramoto I, Kawano S, Kawai S . Gray matter of the bovine cervical spinal cord is mechanically more rigid and fragile than the white matter. J Neurotrauma 2001; 18: 361–367.
Neuhuber B, Barshinger AL, Paul C, Shumsky JS, Mitsui T, Fischer I . Stem cell delivery by lumbar puncture as a therapeutic alternative to direct injection into injured spinal cord. J Neurosurg Spine 2008; 9: 390–399.
Saito F, Nakatani T, Iwase M, Maeda Y, Murao Y, Suzuki Y et al. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: a pilot study. Restor Neurol Neurosci 2012; 30: 127–136.
Callera F, do Nascimento RX . Delivery of autologous bone marrow precursor cells into the spinal cord via lumbar puncture technique in patients with spinal cord injury: a preliminary safety study. Exp Hematol 2006; 34: 130–131.
Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B . Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine 2009; 34: 328.
Nishida K, Tanaka N, Nakanishi K, Kamei N, Hamasaki T, Yanada S et al. Magnetic targeting of bone marrow stromal cells into spinal cord: through cerebrospinal fluid. Neuroreport 2006; 17: 1269–1272.
Bhanot Y, Rao S, Ghosh D, Balaraju S, Radhika CR, Satish Kumar KV . Autologous mesenchymal stem cells in chronic spinal cord injury. Br J Neurosurg 2011; 25: 516–522.
Yoon S, Shim Y, Park Y, Chung J, Nam J, Kim M et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells 2007; 25: 2066–2073.
Lammertse D, Tuszynski M, Steeves J, Curt A, Fawcett J, Rask C et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord 2006; 45: 232–242.
Fawcett J, Curt A, Steeves J, Coleman W, Tuszynski M, Lammertse D et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2006; 45: 190–205.
Wirth B, van Hedel HJ, Kometer B, Dietz V, Curt A . Changes in activity after a complete spinal cord injury as measured by the Spinal Cord Independence Measure II (SCIM II). Neurorehabil Neural Repair 2008; 22: 145–153.
Kim HS, Jeong HJ, Kim MO . Changes of functional outcomes according to the degree of completeness of spinal cord injury. Ann Rehabil Med 2014; 38: 335–341.
Kishk NA, Gabr H, Hamdy S, Afifi L, Abokresha N, Mahmoud H et al. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair 2010; 24: 702–708.
Macias MY, Syring MB, Pizzi MA, Crowe MJ, Alexanian AR, Kurpad SN . Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury. Exp Neurol 2006; 201: 335–348.
Sx Z, Huang F, Gates M, Holmberg EG . Role of endogenous Schwann cells in tissue repair after spinal cord injury. Neural Regen Res 2013; 8: 177.
Bunge MB . Novel combination strategies to repair the injured mammalian spinal cord. J Spinal Cord Med 2008; 31: 262–269.
Ding P, Yang Z, Wang W, Wang J, Xue L . Transplantation of bone marrow stromal cells enhances infiltration and survival of CNP and Schwann cells to promote axonal sprouting following complete transection of spinal cord in adult rats. Am J Transl Res 2014; 6: 224–235.
Acknowledgements
We are grateful to Lida Langroudi from Department of Cell and Systems Biology at University of Toronto for final edition and Mahsa Babaee from Functional Neurosurgery Research Center for helping with manuscript preparation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Oraee-Yazdani, S., Hafizi, M., Atashi, A. et al. Co-transplantation of autologous bone marrow mesenchymal stem cells and Schwann cells through cerebral spinal fluid for the treatment of patients with chronic spinal cord injury: safety and possible outcome. Spinal Cord 54, 102–109 (2016). https://doi.org/10.1038/sc.2015.142
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sc.2015.142
This article is cited by
-
Revolutionizing dermatology: harnessing mesenchymal stem/stromal cells and exosomes in 3D platform for skin regeneration
Archives of Dermatological Research (2024)
-
Stem Cell Therapy in Spinal Cord Injury-Induced Neurogenic Lower Urinary Tract Dysfunction
Stem Cell Reviews and Reports (2023)
-
Clinical translation of stem cell therapy for spinal cord injury still premature: results from a single-arm meta-analysis based on 62 clinical trials
BMC Medicine (2022)
-
Safety and feasibility of autologous olfactory ensheathing cell and bone marrow mesenchymal stem cell co-transplantation in chronic human spinal cord injury: a clinical trial
Spinal Cord (2022)
-
Clinical application of stem cell therapy in neurogenic bladder: a systematic review and meta-analysis
International Urogynecology Journal (2022)