Abstract
Study design:
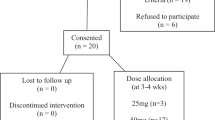
It is a retrospective chart analysis.
Objectives:
In patients with neurogenic lower urinary tract dysfunction (NLUTD) due to spinal cord injury (SCI), neurogenic detrusor overactivity (NDO) can cause both deterioration of the upper urinary tract and urinary incontinence. Antimuscarinic treatment is frequently discontinued due to side effects or lack of efficacy, whereas injection of onabotulinumtoxin into the detrusor is a minimally invasive procedure with risks of urinary retention, infection and haematuria. Mirabegron, a new β-3 agonist, is a potential new agent for treatment of NDO. Aim of the study was to evaluate the efficacy of mirabegron in SCI patients with NLUTD.
Setting:
Swiss Paraplegic Center, Nottwil, Switzerland.
Methods:
A retrospective chart analysis of SCI patient treated with mirabegron.
Results:
Fifteen patients with NDO were treated with mirabegron for a period of at least 6 weeks. Significant reduction of the frequency of bladder evacuation per 24 h (8.1 vs 6.4, P=0.003), and of incontinence episodes per 24 h (2.9 vs 1.3, P=0.027) was observed. Furthermore, we observed improvements in bladder capacity (from 365 to 419 ml), compliance (from 28 to 45 ml cm−1 H20) and detrusor pressure during storage phase (45.8 vs 30 cm H20). At follow-up, 9/15 patients were satisfied with the therapy, 4/15 reported side effects (3 × aggravation of urinary incontinence, 1 × constipation).
Conclusions:
Mirabegron may evolve as an alternative in the treatment of NDO. We observed improvements in urodynamic and clinical parameters. Due to the limited number of patients and the retrospective nature of the study, prospective, placebo-controlled studies are necessary.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Panicker JN, De Seze M, Fowler CJ . Neurogenic lower urinary tract dysfunction. Handb Clin Neurology 2013; 110: 209–220.
Panicker JN, de Seze M, Fowler CJ . Rehabilitation in practice: neurogenic lower urinary tract dysfunction and its management. Clin rehabil 2010; 24: 579–589.
Fowler CJ, Dalton C, Panicker JN . Review of neurologic diseases for the urologist. Urol Clin North Am 2010; 37: 517–526.
Stohrer M, Blok B, Castro-Diaz D, Chartier-Kastler E, Del Popolo G, Kramer G et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Europ Urol 2009; 56: 81–88.
Kessler TM, Bachmann LM, Minder C, Lohrer D, Umbehr M, Schunemann HJ et al. Adverse event assessment of antimuscarinics for treating overactive bladder: a network meta-analytic approach. PLoS ONE 2011; 6: e16718.
Sanford M . OnabotulinumtoxinA (Botox((R))): a review of its use in the treatment of urinary incontinence in patients with multiple sclerosis or subcervical spinal cord injury. Drugs 2014; 74: 1659–1672.
Groen J, Pannek J, Castro Diaz D, Del Popolo G, Gross T, Hamid R et al. Summary of European Association of Urology (EAU) Guidelines on Neuro-Urology. Europ Urol 2015 e-pub ahead of print 21 August 2015; doi: 10.1016/j.eururo.2015.07.071.
Chapple CR, Kaplan SA, Mitcheson D, Blauwet MB, Huang M, Siddiqui E et al. Mirabegron 50 mg once-daily for the treatment of symptoms of overactive bladder: an overview of efficacy and tolerability over 12 weeks and 1 year. Int J Urol 2014; 21: 960–967.
Chapple C, Khullar V, Nitti VW, Frankel J, Herschorn S, Kaper M et al. Efficacy of the beta3-adrenoceptor agonist mirabegron for the treatment of overactive bladder by severity of incontinence at baseline: a post hoc analysis of pooled data from three randomised phase 3 trials. Europ Urol 2015; 67: 11–14.
Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC . Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn 2014; 33: 17–30.
Wada N, Okazaki S, Kobayashi S, Hashizume K, Kita M, Matsumoto S et al. Efficacy of combination therapy with mirabegron for anticholinergic-resistant neurogenic bladder: videourodynamic evaluation. Hinyokika Kiyo 2015; 61: 7–11.
Matsukawa Y, Takai S, Funahashi Y, Yamamoto T, Gotoh M . Urodynamic evaluation of the efficacy of mirabegron on storage and voiding functions in women with overactive bladder. Urology 2015; 85: 786–790.
Yamaguchi O, Kakizaki H, Homma Y, Igawa Y, Takeda M, Nishizawa O et al. Safety and efficacy of mirabegron as 'add-on' therapy in patients with overactive bladder treated with solifenacin: a post-marketing, open-label study in Japan (MILAI study). BJU Int 2015; 116: 612–622.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Wöllner, J., Pannek, J. Initial experience with the treatment of neurogenic detrusor overactivity with a new β-3 agonist (mirabegron) in patients with spinal cord injury. Spinal Cord 54, 78–82 (2016). https://doi.org/10.1038/sc.2015.195
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sc.2015.195
This article is cited by
-
Onabotulinum toxin A improves neurogenic detrusor overactivity following spinal cord injury: a systematic review and meta-analysis
Spinal Cord (2024)
-
Optimal Management of Neurogenic Bladder due to Spinal Cord Injury in Pediatric Patients
Current Bladder Dysfunction Reports (2023)
-
Efficacy and safety of mirabegron for treatment of neurogenic detrusor overactivity in adults with spinal cord injury or multiple sclerosis: a systematic review
Spinal Cord (2022)
-
Urological Care After Spinal Cord Injury
Current Physical Medicine and Rehabilitation Reports (2022)