Abstract
Study design:
Experimental study.
Objectives:
To study the role of surface temperature as an adjunct to motor evoked potentials (MEPs) in rabbit spinal cord injury (SCI) model.
Setting:
Department of Orthopedics, Korea University Guro Hospital, Seoul, Korea.
Methods:
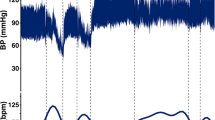
Rabbits (n=18) were divided into Complete (n=9) and Incomplete (n=9) SCI groups. Complete SCI was defined as being non-responsive to a wake-up test with loss of MEPs after transection of spinal cord. Incomplete SCI was defined as being responsive to a wake-up test with significant attenuation (⩾80%) of MEPs after impaction on spinal cord. Surface temperature of upper and lower extremities, core temperature and MEPs signals were checked before, during and after SCI for 20 min. A wake-up test was conducted and spinal cord was histologicaly evaluated.
Results:
Experimental conditions between the two groups were statistically similar (P>0.005 for all values). After SCI, upper extremity temperatures did not change in either group (P>0.005); however, the surface temperature of the lower extremities in the Complete SCI Group elevated to 1.7±0.5 °C in comparison to 0.5±0.1 °C in the Incomplete SCI Group (P<0.001). The scores of wake-up test in the Incomplete SCI Group were significantly different from that of the Complete SCI Group (P<0.001), while white and gray matter damage was variable on histology.
Conclusions:
Monitoring of changes of body surface temperature of the lower extremities can be potentially used to identify the completeness of SCI in a rabbit model.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Malhotra NR, Shaffrey CI . Intraoperative electrophysiological monitoring in spine surgery. Spine 2010; 35: 2167–2179.
Balvin MJ, Song KM, Slimp JC . Effects of anesthetic regimens and other confounding factors affecting the interpretation of motor evoked potentials during pediatric spine surgery. Am J Electroneurodiagnostic Technol 2010; 50: 219–244.
Hong JY, Suh SW, Modi HN, Hur CY, Song HR, Park JH . False negative and positive motor evoked potentials in one patient: is single motor evoked potential monitoring reliable method? A case report and literature review. Spine 2010; 35: E912–E916.
Modi HN, Suh SW, Yang JH, Yoon JY . False-negative transcranial motor-evoked potentials during scoliosis surgery causing paralysis: a case report with literature review. Spine 2009; 34: E896–E900.
Bridwell KH, Dewald RL . The Textbook of Spinal Surgery, 3rd edn Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA. 2011.
Langeloo DD, Journee HL, de Kleuver M, Grotenhuis JA . Criteria for transcranial electrical motor evoked potential monitoring during spinal deformity surgery A review and discussion of the literature. Neurophysiol Clin 2007; 37: 431–439.
Niederhofer H . Effectiveness of the repetitive Transcranical Magnetic Stimulation (rTMS) of 1Hz for Attention-Deficit Hyperactivity Disorder (ADHD). Psychiatr Danub 2008; 20: 91–92.
Meert TF, Vermeirsch HA . A preclinical comparison between different opioids: antinociceptive versus adverse effects. Pharmacol Biochem Behav 2005; 80: 309–326.
Kinoshita H, Yahagi S, Kasai T . Preparatory suppression of the human primary motor cortex induced by repetition of simple and choice reaction time tasks: a transcranial magnetic stimulation study. Brain Res 2007; 1184: 132–140.
MacDonald DB . Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol 2002; 19: 416–429.
Laird AS, Carrive P, Waite PM . Cardiovascular and temperature changes in spinal cord injured rats at rest and during autonomic dysreflexia. J Physiol 2006; 577: 539–548.
Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V . Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma 2000; 17: 1–17.
Yu K, Li J, Jia L, Bao J, Yuan W, Ye T et al. [The effects of graded spinal cord injuries on transcranial electric stimulation motor evoked potentials in the rat]. Zhonghua Wai Ke Za Zhi 1998; 36: 417–420.
Reece TB, Davis JD, Okonkwo DO, Maxey TS, Ellman PI, Li X et al. Adenosine A2A analogue reduces long-term neurologic injury after blunt spinal trauma. J Surg Res 2004; 121: 130–134.
Docherty B, Foudy C . Homeostasis part 3: temperature regulation. Nurs Times 2006; 102: 20–21.
Tarlov IM, Klinger H . Spinal cord compression studies. II. Time limits for recovery after acute compression in dogs. AMA Arch Neurol Psychiatry 1954; 71: 271–290.
Basso DM, Beattie MS, Bresnahan JC . Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 1996; 139: 244–256.
Faul F, Erdfelder E, Buchner A, Lang AG . Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Beh Res Methods 2009; 41: 1149–1160.
White RJ . Pathology of spinal cord injury in experimental lesions. Clin Orthop Relat Res 1975; 112: 16–26.
Popa C, Popa F, Grigorean VT, Onose G, Sandu AM, Popescu M et al. Vascular dysfunctions following spinal cord injury. J Med Life 2010; 3: 275–285.
Roehl K, Becker S, Fuhrmeister C, Teuscher N, Futing M, Heilmann A . New, non-invasive thermographic examination of body surface temperature on tetraplegic and paraplegic patients, as a supplement to existing diagnostic measures. Spinal Cord 2009; 47: 492–495.
Onifer SM, Rabchevsky AG, Scheff SW . Rat models of traumatic spinal cord injury to assess motor recovery. ILAR J 2007; 48: 385–395.
Acknowledgements
This study was supported by a grant of the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI11C0388). This study was performed under the approval of the IACUC (Institutional Animal Care and Use Committee, KU12129). All experimental procedures, protocols and treatment of experimental animals were performed under the control of the IACUC. This study was performed in the Korea University Guro Hospital.
Author contributions
SWS carried out the arrangement of the enrolled patients and drafted the manuscript as an assistant of JHY. YSP participated in the design of the study and performed the statistical analysis as an assistant of JHY. SWS carried out the arrangement of the enrolled patients and drafted the manuscript as an assistant of JHY. J-HL and BKP participated in the design of the study. CHH participated in the design and revision work of the study. JHY participated in the design of the study, statistical analysis and coordination, and drafted the manuscript as the main author. All authors read and approved the final manuscript.
Disclaimer
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yang, J., Suh, S., Park, YS. et al. Change in body surface temperature as an ancillary measurement to motor evoked potentials. Spinal Cord 53, 827–834 (2015). https://doi.org/10.1038/sc.2015.90
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sc.2015.90