Abstract
Objectives:
This study aimed to investigate the molecular mechanisms underlying the positive role of treadmill training (TMT) in locomotor recovery.
Methods:
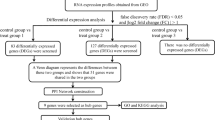
GSE52763 microarray data were downloaded from GEO database, which was collected from the lumbar spinal cord samples of three groups of mice: mice subjected to contusive injury and killed 1 week after injury (I1), mice subjected to injury and killed 3 weeks after injury (I3), and mice subjected to injury and TMT beginning at week 1 and lasting until week 3 (T3). Differential expression analysis between I3 and I1, between T3 and I1 and between T3 and I3 were performed by T-test using R/LIMMA. Genes with |log2FC (fold change)|>0.58 and P-value<0.05 were considered as differentially expressed genes (DEGs). Specific I3 vs I1 DEGs and T3 vs I1 DEGs were screened. Then TMT-induced specific DEGs were subject to functional and pathway enrichment analysis using DAVID online tool. Protein–protein interaction (PPI) analysis was also carried out using the STRING database.
Results:
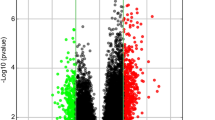
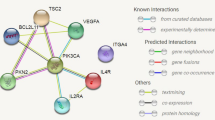
Finally, 82 upregulated DEGs and 297 downregulated DEGs were found specifically induced by TMT. Specific upregulated DEGs were mostly enriched in response to organic substance and morphogenesis-related events, and specific downregulated DEGs were related to positive regulation of transcription. ATP2A1, PRKACA, ITPR2 and so on had high connection degree in the PPI network of the specific upregulated DEGs; FOS, GSK3B and so on had high degrees in the PPI network of the specific downregulated DEGs.
Conclusion:
ATP2A1, C-FOS and GSK3B may have critical roles in the positive role of TMT in locomotor recovery.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Oliveri RS, Bello S, Biering-Sørensen F . Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: systematic review with meta-analyses of rat models. Neurobiol Dis 2014; 62: 338–353.
Forssberg H, Grillner S, Halbertsma J . The locomotion of the low spinal cat I. Coordination within a hindlimb. Acta physiol Scand 1980; 108: 269–281.
Belanger M, Drew T, Provencher J, Rossignol S . A comparison of treadmill locomotion in adult cats before and after spinal transection. J Neurophysiol 1996; 76: 471–491.
Leblond H, L'Espérance M, Orsal D, Rossignol S . Treadmill locomotion in the intact and spinal mouse. J Neurosci 2003; 23: 11411–11419.
Barbeau H, Rossignol S . Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 1987; 412: 84–95.
Battistuzzo CR, Callister RJ, Callister R, Galea MP . A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J Neurotrauma 2012; 29: 1600–1613.
Shin HY, Kim H, Kwon MJ, Hwang DH, Lee K, Kim BG . Molecular and cellular changes in the lumbar spinal cord following thoracic injury: regulation by treadmill locomotor training. PLoS ONE 2014; 9: e88215.
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003; 4: 249–264.
Gautier L, Cope L, Bolstad BM, Irizarry RA . Affy–-analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 2004; 20: 307–315.
Diboun I, Wernisch L, Orengo CA, Koltzenburg M . Microarray analysis after RNA amplification can detect pronounced differences in gene expression using LIMMA. BMC Genomics 2006; 7: 252.
Oliveros JC An interactive tool for comparing lists with Venn Diagrams. VENNY, 2007. http://bioinfogp.cnb.csic.es/tools/venny/index.html.
Huang DW, Sherman BT, Lempicki RA . Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2008; 4: 44–57.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM et al. Gene Ontology: tool for the unification of biology. Nat Genet 2000; 25: 25–29.
Aoki-Kinoshita KF, Kanehisa M Gene annotation and pathway mapping in KEGG Methods Mol Biol 2007; 396: 71–91.
Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A et al. STRING v9. 1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res 2013; 41: D808–D815.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003; 13: 2498–2504.
Odermatt A, Taschner PE, Khanna VK, Busch HF, Karpati G, Jablecki CK et al. Mutations in the gene–encoding SERCA1, the fast–twitch skeletal muscle sarcoplasmic reticulum Ca2+ ATPase, are associated with Brody disease. Nat Genet 1996; 14: 191–194.
Cai J, Cheng A, Luo Y, Lu C, Mattson MP, Rao MS et al. Membrane properties of rat embryonic multipotent neural stem cells. J Neurochem 2004; 88: 212–226.
Banday AR, Azim S, Hussain MA, Nehar S, Tabish M . Computational prediction and characterisation of ubiquitously expressed new splice variant of Prkaca gene in mouse. Cell Biol Int 2013; 37: 687–693.
Meyer MA . Identification of 17 highly expressed genes within mouse lumbar spinal cord anterior horn region from an in-situ hybridization atlas of 3430 genes: implications for motor neuron disease. Neurol Int 2014; 6: 5367.
Yamamoto-Hino M, Sugiyama T, Hikichi K, Mattei M, Hasegawa K, Sekine S et al. Cloning and characterization of human type 2 and type 3 inositol 1, 4, 5-trisphosphate receptors. Receptors Channels 1993; 2: 9–22.
Mikoshiba K . Inositol 1, 4, 5‐trisphosphate (IP3) receptors and their role in neuronal cell function. J Neurochem 2006; 97: 1627–1633.
Van Den Bosch L, Verhoeven K, De Smedt H, Wuytack F, Missiaen L, Robberecht W . Calcium handling proteins in isolated spinal motoneurons. Life Sci 1999; 65: 1597–1606.
Cleveland DW, Rothstein JD . From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2001; 2: 806–819.
Kerppola T, Curran T . Selective DNA bending by a variety of bZIP proteins. Mol Cell Biol 1993; 13: 5479–5489.
Karin M, Liu Z-g, Zandi E . AP-1 function and regulation. Curr Opin Cell Biol 1997; 9: 240–246.
Shaulian E, Karin M . AP-1 as a regulator of cell life and death. Nat Cell Biol 2002; 4: E131–E136.
Dragunow M, Faull R . The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 1989; 29: 261–265.
Bullitt E . Expression of C‐fos‐like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol 1990; 296: 517–530.
Munglani R, Hunt SP . Proto-oncogenes: basic concepts and stimulation induced changes in the spinal cord. Prog Brain Res 1995; 104: 283–298.
Morgan JI, Curran T . Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci 1991; 14: 421–451.
Hudspith M, Harrisson S, Smith G, Bountra C, Elliot P, Birch P et al. Effect of post-injury NMDA antagonist treatment on long-term Fos expression and hyperalgesia in a model of chronic neuropathic pain. Brain Res 1999; 822: 220–227.
Catheline G, Le Guen S, Honoré P, Besson J-M . Are there long-term changes in the basal or evoked Fos expression in the dorsal horn of the spinal cord of the mononeuropathic rat? Pain 1999; 80: 347–357.
Kajander KC, Madsen AM, Iadarola MJ, Draisci G, Wakisaka S . Fos-like immunoreactivity increases in the lumbar spinal cord following a chronic constriction injury to the sciatic nerve of rat. Neurosci Lett 1996; 206: 9–12.
Seira O, Gavín R, Gil V, Llorens F, Rangel A, Soriano E et al. Neurites regrowth of cortical neurons by GSK3β inhibition independently of Nogo receptor 1. J Neurochem 2010; 113: 1644–1658.
Dill J, Wang H, Zhou F, Li S . Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci 2008; 28: 8914–8928.
Alabed YZ, Pool M, Tone SO, Sutherland C, Fournier AE . GSK3β regulates myelin-dependent axon outgrowth inhibition through CRMP4. J Neurosci 2010; 30: 5635–5643.
Parkitna JR, Obara I, Wawrzczak-Bargiela A, Makuch W, Przewlocka B, Przewlocki R . Effects of glycogen synthase kinase 3β and cyclin-dependent kinase 5 inhibitors on morphine-induced analgesia and tolerance in rats. J Pharmacol Exp Ther 2006; 319: 832–839.
Yang M, Li J, So K, Chen J, Cheng W, Wu J et al. Efficacy and safety of lithium carbonate treatment of chronic spinal cord injuries: a double-blind, randomized, placebo-controlled clinical trial. Spinal Cord 2012; 50: 141–146.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, Q., Zhang, B., Liu, C. et al. Molecular mechanisms underlying the positive role of treadmill training in locomotor recovery after spinal cord injury. Spinal Cord 55, 441–446 (2017). https://doi.org/10.1038/sc.2016.134
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sc.2016.134
This article is cited by
-
Osteopontin enhances the effect of treadmill training and promotes functional recovery after spinal cord injury
Molecular Biomedicine (2023)
-
Identification of the potential regulatory interactions in rheumatoid arthritis through a comprehensive analysis of lncRNA-related ceRNA networks
BMC Musculoskeletal Disorders (2023)