Abstract
Study design:
Retrospective statistical analysis of database.
Objective:
Spinal cord injury (SCI) clinical trials are challenged to enroll participants, and early trial outcomes have often been equivocal. We hypothesized that a specifically designed novel true linear interval-scaled outcome measure targeted to simultaneously track a broad range of SCI will enable more inclusive enrollment of participants and valid comparisons of functional changes after SCI.
Methods:
To define a single SCI measurement framework, we used items from existing measures. To evaluate linearity and validity of the measure, we used rigorous psychometric Rasch analysis on two data sets from over 2500 traumatic SCI participants (all levels and severities of SCI) within the EMSCI (European Multicenter study about SCI) database.
Results:
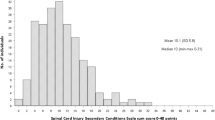
Volitional performance was found to be the unidimensional construct that would detect and track a treatment effect from a central nervous system-directed therapeutic. Along with early evidence for voluntary neurological control of upper-extremity muscle contractions, volitional performance is best described by goal-directed activities of daily living that are increasingly difficult to re-acquire when activity within more caudal spinal segments is required. Validity of the Spinal Cord Ability Ruler (SCAR) as a linear interval construct was confirmed with Rasch analysis. All measurement items were properly ordered, as well as being precise and stable across clinically relevant groups. Only 5/24 items had some misfit. Targeting was excellent over time after SCI, with few gaps and only modest floor and ceiling effects (3% each).
Conclusions:
SCAR is a quantitative linear measure of volitional performance across an inclusive range of tetraplegic and paraplegic SCI.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Spinal Cord Injury (SCI) Facts and Figures at a Glance. J Spinal Cord Med 2015; 38: 249–250.
Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, Laramee MT et al. A multicenter international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord 2007; 45: 275–291.
Bluvshtein V, Front L, Itzkovich M, Benjamini Y, Galili T, Gelernter I et al. A new grading for easy and concise description of functional status after spinal cord lesions. Spinal Cord 2012; 50: 42–50.
Fromovich-Amit Y, Biering-Sorensen F, Baskov V, Juocevicius A, Hansen HV, Gelernter I et al. Properties and outcomes of spinal rehabilitation units in four countries. Spinal Cord 2009; 47: 597–603.
Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med 2011; 34: 547–554.
Medical Research Council Aids to the Examination of the Peripheral Nervous System, Memorandum No. 45. Her Majesty’s Stationery Office: London, UK. 1981.
Nesathurai S . Steroids and spinal cord injury: revisiting the NASCIS 2 and NASCIS 3 trials. J Trauma 1998; 45: 1088–1093.
Marino RJ, Graves DE . Metric properties of the ASIA motor score: subscales improve correlation with functional activities. Arch Phys Med Rehabil 2004; 85: 1804–1810.
Anderson KD, Acuff ME, Arp BG, Backus D, Chun S, Fisher K et al. United States (US) multi-center study to assess the validity and reliability of the Spinal Cord Independence Measure (SCIM III). Spinal Cord 2011; 49: 880–805.
Itzkovich M, Tripolski M, Zeilig G, Ring H, Rosentul N, Ronen J et al. Rasch analysis of the Catz-Itzkovich spinal cord independence measure. Spinal Cord 2002; 40: 396–407.
Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2007; 45: 190–205.
Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 2007; 45: 206–221.
Tanadini LG, Steeves JD, Hothorn T, Abel R, Maier D, Schubert M et al. Identifying homogeneous subgroups in neurological disorders: unbiased recursive partitioning in cervical complete spinal cord injury. Neurorehabil Neural Repair 2014a; 28: 507–515.
Tanadini L, Hothorn T, Jones L, Lammertse DP, Abel R, Maier D et al. Toward inclusive trial protocols in heterogeneous neurological disorders: prediction-based stratification of participants with incomplete cervical spinal cord injury. Neurorehabil Neural Repair 2014b; 29: 867–877.
Hobart JC, Cano SJ, Thompson AJ . Effect size statistics can be misleading: is it time to change the way we measure change? J Neurol Neurosurg Psychiatry 2010; 81: 1044–1048.
Hobart JC, Cano SJ, Zajicek JP, Thompson AJ . Rating scales as outcome measures for clinical trials in neurology: problems, solutions, and recommendations. Lancet 2007; 6: 1094–1105.
Pouw MH, Van Middendorp JJ, Van Kampen A, Curt A, Van De Meent H, Hosman AJ . Diagnostic criteria of traumatic central cord syndrome. Part 3: descriptive analyses of neurological and functional outcomes in a prospective cohort of traumatic motor incomplete tetraplegics. Spinal Cord 2011; 49: 614–622.
Curt A, Ellaway PH . Clinical neurophysiology in the prognosis and monitoring of traumatic spinal cord injury. Handb Clin Neurol 2012; 109: 63–75.
Hobart J, Cano S . Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods. Health Technol Assess 2009; 13: iii, ix-x, 1–177.
Belvedere SL, De Morton NA . Application of Rasch analysis in health care is increasing and is applied for variable reasons in mobility instruments. J Clin Epidemiol 2010; 63: 1287–1297.
Andrich D, Sheridan B, Luo G . Perth: RUMM Laboratory; 2030. 2008. Rasch Unidimensional Measurement Model. Computer software program.
Lindeboom R, Vermeulen M, Holman R, De Haan RJ . Activities of daily living instruments: Optimizing scales for neurologic assessments. Neurology 2003; 60: 738–742.
Guyenet PG . Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 2014; 4: 1511–1562.
Drake MJ . Management and rehabilitation of neurologic patients with lower urinary tract dysfunction. Handb Clin Neurol 2015; 130: 451–468.
Weng LJ . Impact of the number of response categories and anchor labels on coefficient alpha and test-retest reliability. Educ Psych Measure 2004; 64: 956–972.
Alexander MS, Anderson K, Biering-Sorensen F, Blight AR, Brannon R, Bryce TN et al. Outcome measures in spinal cord injury: recent assessments and recommendations for future directions. Spinal Cord 2009; 47: 582–591.
Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1—eliciting concepts for a new PRO instrument. Value Health 2011; 14: 967–977.
Wu X, Liu J, Tanadini LG, Lammertse DP, Blight AR, Kramer JL et al. Challenges for defining minimal clinically important difference (MCID) after spinal cord injury. Spinal Cord 2015; 53: 84–91.
Acknowledgements
We are grateful to Asubio Pharma for their support, which aided some of the statistical analysis. We also appreciate the efforts of René Koller, EMSCI database manager. We acknowledge support provided by EMSCI (funded by IRP Switzerland) and SCOPE for facilitating meetings and guiding discussions to formulate the SCAR concept.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Reed, R., Mehra, M., Kirshblum, S. et al. Spinal cord ability ruler: an interval scale to measure volitional performance after spinal cord injury. Spinal Cord 55, 730–738 (2017). https://doi.org/10.1038/sc.2017.1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sc.2017.1
This article is cited by
-
Minimal clinically important difference (MCID) and minimal detectable change (MDC) of Spinal Cord Ability Ruler (SCAR)
Spinal Cord (2023)
-
Standardized administration and scoring guidelines for the Spinal Cord Independence Measure Version 3.0 (SCIM-III)
Spinal Cord (2023)
-
Baseline-adjusted proportional odds models for the quantification of treatment effects in trials with ordinal sum score outcomes
BMC Medical Research Methodology (2020)
-
Feasibility of predicting improvements in motor function following SCI using the SCAR outcome measure: a retrospective study
Spinal Cord (2019)
-
The challenge of recruitment for neurotherapeutic clinical trials in spinal cord injury
Spinal Cord (2019)