Figure 5
From: Hydrogen reduction of molybdenum oxide at room temperature

Left: Sketch of the potential energy surface for the reduction of MoO3 to MoO2 to depict the possible reaction pathways. The z-axis is the energy coordinate, while x- and y-axes represent the not further defined reaction coordinates. MoO2 is most stable with respect to MoO3 and hydrogen, however, hydrogen bronze HxMoO3 is a metastable state. The corresponding enthalpies of formations serve as quantitative estimates for the position on the energy potential surface26. The activation energy of the reactions depends on the path over the potential energy surface. The corresponding experimental activation energies EA for the high temperature reduction are indicated15. Hydrogen diffusion is relatively fast in MoO3, which results from the small activation energy for diffusion 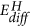 29. The latter is related to the electronic structure on one hand, but the reaction path is also affected by the external conditions temperature, hydrogen and water partial pressure on the other. These conditions depend on the experimental setup as sketched in the right figures: catalysts are usually made of MoO3 particles, which are basically inert at room temperature, i.e., they do not dissociate hydrogen nor is desorption of water facilitated (bottom left). At higher temperature, an oxygen deficient, dissociatively active surface is formed with high oxygen mobility (bottom right). To overcome the high dissociation at room temperature, one can coat a MoO3 thin film on an inert substrate with Pd (typical sensor setup, top left). In our membrane approach, the MoO3 thin film is hydrogenated via the Pd substrate, i.e., potentially formed water can leave the film.
29. The latter is related to the electronic structure on one hand, but the reaction path is also affected by the external conditions temperature, hydrogen and water partial pressure on the other. These conditions depend on the experimental setup as sketched in the right figures: catalysts are usually made of MoO3 particles, which are basically inert at room temperature, i.e., they do not dissociate hydrogen nor is desorption of water facilitated (bottom left). At higher temperature, an oxygen deficient, dissociatively active surface is formed with high oxygen mobility (bottom right). To overcome the high dissociation at room temperature, one can coat a MoO3 thin film on an inert substrate with Pd (typical sensor setup, top left). In our membrane approach, the MoO3 thin film is hydrogenated via the Pd substrate, i.e., potentially formed water can leave the film.
