Abstract
Gene expression studies were undertaken in normal pancreas and pancreatic adenocarcinomas to determine new candidate genes that can potentially be used as markers of the disease. The characteristic desmoplastic stromal reaction of pancreatic adenocarcinoma greatly hampers expression studies in this tumour type, and usually necessitates time-consuming tissue microdissection for enrichment of the tumour cell population. We show that fine needle aspiration of cancer provides a fast and efficient way of obtaining samples highly enriched in tumour cells with sufficient yields of RNA. Using Atlas cancer cDNA arrays with 588 cancer-related genes, we describe gene expression profiles of normal pancreas, bulk pancreatic tumour tissues and pancreatic tumour aspirates containing more than 95% tumour cells. Analysis of bulk tissue specimens revealed differentially expressed genes belonging predominantly to the stromal component of the tumour. This contrasted with the results obtained from tumour-cell enriched samples. Several genes already described in pancreatic cancer (caspase 8, TIMP1, CD9, IL-13) were also differentially expressed in our study. Furthermore, we found dysregulated expression of genes not previously associated with pancreatic adenocarcinoma, such as Rac 1, GLG1, NEDD5, RPL-13a, RPS9 and members of the Wnt5A gene family. In summary, we present a panel of genes newly identified in the pathogenesis of pancreatic adenocarcinoma and demonstrate that fine needle aspirates of the tumour mass are a convenient source of material for gene expression studies in tumours accompanied by desmoplastic reactions.
Similar content being viewed by others
Introduction
Large scale gene expression profiling using oligonucleotide or cDNA arrays has become one of the most fruitful methods for characterizing physiological and pathological processes. It has been used in a variety of applications, from annotation of the human genome to tumour classification that might predict prognosis and response to treatment (Alizadeh et al., 2000; Golub et al., 1999; Perou et al., 2000; Ross et al., 2000; Shoemaker et al., 2001).
Pancreatic adenocarcinoma poses particular problems for gene expression studies. This malignancy is characterized by a dense desmoplastic stromal component, with neoplastic cells often representing a minor population in many parts of the tumour mass. To date, two different approaches have been used to compensate for the dilution of the neoplastic element. One previous study (Gress et al., 1996) used hybridization to filters carrying cDNA clones derived from pancreatic cancer cell lines in an attempt to restrict the expression profile to genes more likely to be derived from the malignant epithelial component of the tumour. The other used a mixture of RNAs from chronic pancreatitis and health pancreas as the driver population in representational difference analysis (Gress et al., 1997). Recently, the laser-capture microdissection technique for procuring individual components of complex tissues has been designed (Emmert-Buck et al., 1996; Sirivatanauksorn et al., 1999), but has proven to be labour-intensive and time-consuming. In addition, the amount of material generated by microdissection is limited and, without amplification procedures, usually not sufficient for gene expression studies. In this study we have therefore explored an alternative approach for enrichment of neoplastic cells by using fine needle aspiration from the tumour mass. We show that it is a practical and convenient approach for generating suitable source material with high quality and quantity of nucleic acids for downstream molecular analysis.
Adenocarcinoma of the exocrine pancreas is the most common type of pancreatic malignancy. It often presents symptoms only when it is already in an advanced state and curative resection is no longer possible. Detailed knowledge of genes involved in the growth of pancreatic adenocarcinoma might enable construction of molecular tests for earlier diagnosis (Lemoine, 1997) and identify new targets for therapy (Aspinall and Lemoine, 1999). We have therefore examined expression profiles in both the enriched malignant epithelial component of the tumour and in the bulk cancerous tissue consisting predominantly of stromal elements in order to build up a more complete picture of the network of events involved in pancreatic tumorigenesis.
Results
Analysis of bulk tumours including stromal components
Gene expression in bulk adenocarcinoma samples was compared to that of normal pancreas. Differentially expressed genes that are common in hybridization with all six bulk specimens vs normal pancreas are listed in Table 1. The highest upregulation was displayed by collagen, vimentin and decorin, and the most marked downregulation by ribosomal protein L13a and interleukin 13.
Analysis of samples selectively enriched for tumour component
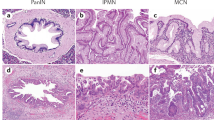
Enrichment of the samples with tumour cells was achieved by the fine needle aspiration technique described in Materials and methods, which produced samples containing around 95% cytokeratin-positive tumour cells (Figure 1).
(a) Histopathological appearance of a human pancreatic cancer showing a cancer cellularity of about 35%; (b) the fine needle aspirate obtained in this case, where cancer cells comprise more than 95% of the total cell population. (Hematoxylin and eosin staining, original magnification ×20); (c) Immunocytochemical staining of cellular aspirate for cytokeratin 8/18; (d) SYBRGold-stained agarose gel of three RNA samples derived from the cellular aspirates
Comparative hybridization analysis of tumour cell-enriched samples against normal tissue showed a different pattern of gene expression between the aspirated cancer cells and the normal pancreas (Table 2, Figure 2), that was distinct from the one obtained when comparing bulk tumours with normal tissue (Table 1). Vimentin, decorin and CD9 showed no differential expression after enrichment of samples with tumour cells. Some genes that had differentials below the twofold cut-off in the analysis of bulk tissue samples displayed higher up- or down-regulation in the analysis of the purified cell population (Golgi membrane sialoglycoprotein (MG160/GLG1), matrix metalloproteinase 16, leukocyte interferon-inducible peptide). Several genes already known through candidate gene analysis to be upregulated in pancreatic cancer were confirmed in our analysis, including caspase 8 and tissue inhibitor of matrix metalloproteinase 1 (TIMP1). Some genes showed similar differentials in the analysis of both whole-tissue homogenates and tumour cell aspirates, suggesting that their expression pattern is shared by both the neoplastic cells and their supporting stromal elements. Examples in this class include neural precursor cell expressed/developmentally downregulated 5 (NEDD5, more than twofold upregulated in cancer compared to normal) and interleukin-13 (IL-13, more than fivefold downregulated). Hence by comparing the profile of gene expression in the material of mixed cellularity to that in cancer cells alone, it is also possible to define the contribution of stromal elements. Genes belonging to the Wnt gene family, namely Wnt5A, secreted apoptosis-related proetin-1 (SARP1/hFRP2), dishevelled (DVL) and c-jun N terminal kinase 2 (JNK2) that were previously not associated with pancreatic adenocarcinomas showed deregulated expression as well (Table 2, Figure 2).
Of 187 genes that were expressed in tumour cell-enriched samples (i.e. intensity value higher than twice the background), 149 were found in common with the genes expressed in each of the bulk pancreatic cancer experiments. The full list of those commonly expressed genes that represent the expression profile of pancreatic adenocarcinoma is available as supplementary information on the WWW (http://www.lif.icnet.uk/axp/mphh/Supplementary/index.html).
In hybridizations with the normal donor pancreas, expression of a total of 164 genes was confirmed in at least two experiments. Those genes comprising the gene expression profile of completely healthy pancreas can also be viewed as supplementary information at the same WWW location.
Validation of gene expression data
We have validated differential gene expression by real-time quantitative RT–PCR for two of the genes (Rac 1 and GLG1) and the other eight were confirmed by data from publicly available SAGE studies and UniGene Digital Differential Display (DDD).
Real-time quantitative RT–PCR analysis of Rac 1 and GGL1 expression
We have chosen to study in more detail the ras-related gene Rac 1, knowing the importance of ras and other small GTPases in pancreatic cancer and carcinogenesis in general. Rac 1 was expressed in all experiments with cancer samples and 2.7-fold up-regulated in the first set of three carcinomas (see Table 1 and supplementary data). Quantitative data on Rac 1 expression in normal and malignant pancreatic tissues and pancreatic cancer cell lines are shown in Figure 3. In 14 of 20 (70%) cancer cases studied, Rac 1 was overexpressed more than twofold in comparison to the mean value for completely healthy pancreatic tissues (Npa1 and Npa2 in Figure 3). A slight increase in Rac 1 expression was noticed in sample 4N (matched non-neoplastic tissue to malignant sample Tm4). Histopathologic examination showed that this sample was complicated by a particularly marked chronic pancreatitis, which probably contributed to the Rac 1 overexpression. In eight tumours, Rac 1 was up-regulated from 10-fold (case Tm13) to almost 40-fold (case Tm2). Of note, weak expression of Rac 1 in tumour samples Tm15, Tm16 and Tm17, which constitute the second pool of three bulk specimens corresponds well to their low expression obtained in array hybridization. All pancreatic cancer cell lines displayed even higher Rac 1 expression levels, between 20 and 140 times the level of Rac 1 expression in normal pancreas (note separate scale in Figure 3).
Real-time quantitative RT–PCR data for the GLG1 gene are summarized in Figure 4. More than half of the tumour samples show more than twofold gene up-regulation in comparison to the mean value for completely healthy pancreatic tissues Npa1 and Npa2, with the highest overexpression being more than 10-fold. In the two cases where there were matched specimens from the same patient (Tm4/4N and Tm10/10N), GLG1 expression was more than threefold higher in the tumour tissues. All the six cell lines showed more than 10-fold upregulation of the GLG1 transcript.
SAGE and DDD data
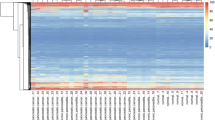
Upregulation of decorin and collagen 3 alpha 1 was confirmed when comparing four pooled pancreatic cancer libraries with five pooled libraries from normal pancreas by Digital Differential Display [http://www.ncbi.nlm.nih.gov/UniGene] (data not shown). Those two genes were also confirmed as differentially expressed in SAGE data (Figure 5). In addition, we identified downregulation of the ribosomal protein genes RPS9 and RPL-13a in cancer, when comparing two bulk pancreatic cancer SAGE libraries with SAGE libraries from normal duct epithelial cells (http://www.ncbi.nlm.nih.gov/SAGE/sagexp.cgi). Differential gene expression of an additional four genes observed in our study was confirmed with SAGE data (Figure 5). Namely, versican, Rho GTPase-activating protein 4 and caveolin-1 were only found expressed in cancer tissue and/or cell lines; and caspase 8 was found to be more highly expressed in pancreatic cell lines than in bulk cancer and normal ducts, which is in good agreement with our finding of its upregulation in only tumour cell-enriched samples (Table 2).
Virtual Northern of genes differentially expressed in SAGE libraries of normal pancreatic ductal cells (HX and H126), bulk pancreatic cancer tissues (Panc91–6113 and Panc96–6252) and pancreatic cancer cell lines (CAPAN1, CAPAN2, HS766T and PANC1). Col3a1: procollagen 3 alpha 1 subunit; RPS9: ribosomal protein S9; RPL-13a: ribosomal protein L-13a VER: versican; ARHGAP4: Rho-GAP activating protein 4; CAV1: caveolin-1; CASP8: caspase 8
Discussion
Fine needle aspiration of cancer for neoplastic cell enrichment
We describe a convenient method of obtaining an enriched population of tumour cells from pancreatic adenocarcinoma, which is a tumour type characterized by a highly desmoplastic stromal reaction. Tissue aspiration is less time-consuming and more straightforward than microdissection techniques and provides RNA samples of good quality and quantity. This is essential for gene expression studies and critical in the analysis of the pancreas, which is an organ with high endogenous nuclease activity. Cell enrichment by aspiration is based on the general characteristic of epithelial tumour cells to have decreased adhesion, therefore allowing easy separation from the surrounding stroma. This technique could potentially be applied to obtain tumour cell-enriched samples from other epithelial tumour types characterized by various levels of desmoplastic reaction.
Differentially expressed genes in bulk neoplastic tissues
The first part of our study addressed genes expressed in bulk specimens of pancreatic cancer, in which the profiles will reflect the mixed composition of the tissue sample. A number of transcripts were expressed in both (see supplementary data at http://www.lif.icnet.uk/axp/mphh/Supplementary/index.html), analogous to the situation described in a SAGE study of normal colon and colon carcinomas (Zhang et al., 1997). As bulk adenocarcinoma samples in general contain not more than 30–40% tumour cells, those samples were expected to display genes predominantly involved in the development of desmoplasia. This phenomenon is an intense stromal fibrosis induced by activation of fibroblasts and pancreatic stellate cells, with increased production of matrix proteins and matrix degrading enzymes (Gress et al., 1998). An accentuated stromal reaction has been described as an independent prognostic factor in a variety of tumour types including breast (Cardone et al., 1997) and colorectal cancer (Halvorsen and Seim, 1989). Desmoplastic stroma typically consists of collagens, proteoglycans and glycoproteins. We describe the involvement of several genes that contribute to the desmoplastic reaction in pancreatic adenocarcinoma, namely collagens of types I and III, decorin and versican. Involvement of collagens has already been well documented (Gress et al., 1995; Ryu et al., 2001).
The small, collagen-associated proteoglycan decorin plays an important role in the remodelling and maintenance of extracellular matrix (ECM) during inflammation, fibrosis and cancer growth through its ability to bind ECM constituents and growth factors like epidermal growth factors and TGFβ, whose involvement in pancreatic adenocarcinoma has been well documented (Asakura et al., 1999; Lohr et al., 2001; Moscatello et al., 1998). Versican is another proteoglycan that was found upregulated in pancreatic cancer. Immunolocalization of both versican and decorin has been described in the peritumoural stroma of early-stage prostate cancer, another tumour type that typically displays a desmoplastic reaction(Ricciardelli et al., 1998).
One additional gene that was found upregulated in bulk cancer tissue in comparison with normal cells was Rac 1. The involvement of various small GTPases has already been described in a number of epithelial tumours. In a recent study, RhoA overexpression was found in the majority of colon, breast and lung carcinomas, proving to be a more general phenomenon than abnormalities of p53, ras and c-myc, each of which were observed in not more than 30–60% of tumours (Fritz et al., 1999). In pancreatic adenocarcinomas, higher expression levels of RhoC have been described and found significantly correlated with poor prognosis (Suwa et al., 1998). We report that Rac 1 (ras-related C3 botulinum toxin substrate) is upregulated in around 70% of tumour samples examined (Table 1 and Figure 3). Rac 1 overexpression was therefore confirmed both by a hybridization approach and by real-time quantitative RT–PCR. In addition to increased levels of expression in malignant pancreatic tissue, Rac 1 expression was extremely high in all six pancreatic cancer cell lines examined (Figure 3). Rac 1 regulates various cell functions such as cell shape, cell motility and cytokinesis through reorganization of the actin cytoskeleton and cross-talk with cadherin-dependent intercellular adhesion (Takaishi et al., 1997). It acts downstream of ras and is critical to ras-induced transformation, but it is also an independent transforming agent. Fibroblasts expressing Rac 1 mutated to valine at position 12 show a marked increase in lamellipodia, increased and anchorage-independent cell proliferation and enhanced tumorigenicity in athymic nude mice (Qiu et al., 1995). In our series of 20 pancreatic adenocarcinoma cases and eight cell lines it appears that there is no connection between K-ras status and Rac 1 expression level (data not shown), which is consistent with a recent study of Rac 1 in breast carcinoma (Schnelzer et al., 2000). In contrast to breast cancer, K-ras activating mutations are present in the majority of pancreatic adenocarcinomas, and so it would be of interest to study the interaction of the two GTPases, as well the potentially independent growth promoting activity of Rac 1 in pancreatic carcinoma. Up-regulation in bulk samples but no differential expression in aspirates would argue for shared stromal and epithelial expression. At present, specific antibodies that distinguish Rac 1 and Rac 2 are not available (the Rac 1 and Rac 2 genes are 92% homologous and both transcripts were obtained in our study), and the exact location of Rac 1 expression remains uncertain.
The most marked downregulation in bulk cancer experiments was displayed by ribosomal protein L13a. A secondary role other than in protein biosynthesis has recently been described for a number of ribosomal proteins. RPL13a, for example, is involved in regulation of cell proliferation (inducing arrest of cell cycle in G2/M phase) and induces apoptosis in leukaemic U937 cells (Chen et al., 1998). Its downregulation shown here is consistent with a potentially similar function in pancreatic cancer cells.
Our study revealed several genes whose involvement in pancreatic exocrine tumours has been previously reported: TIMP1, caspase 8, IL-13 and CD9. Their presence was invaluable as an independent confirmation of the validity of data obtained by array hybridization. CD9 is a ubiquitously expressed tetraspanin that forms complexes with integrins, other tetraspanins and MHC antigens and is associated with small GTP-binding proteins (Berditchevski and Odintsova, 1999). In pancreatic cancer, reduced CD9 expression levels have been associated with high tumour grade and lymph node metastasis (Sho et al., 1998). In our study it was evident that whilst there are elevated levels of CD9 in the stroma (Table 1), there is reduced CD9 expression in the tumour cell population (Table 2). This again points to the necessity of monitoring gene expression profiles in the context of a homogenous population of cells purified from a complex tissue. Interleukin-13 is a pleiotropic cytokine produced by tumour-infiltrating lymphocytes. Its receptor is present on the majority of cancer cells and cell lines (including pancreatic cancer), usually conveying an antiproliferative effect (Kornmann et al., 1999). Interleukin-13 was found downregulated in both bulk tumour samples and aspirates (Tables 1 and 2). TIMP1 and caspase 8 were obtained only after enrichment of samples with tumour cells (Bramhall et al., 1997; Virkajarvi et al., 1998; Zhang et al., 1997).
Differentially expressed genes in cancer cells
The most interesting genes that were found dysregulated in tumour cell-enriched samples were GLG1, leukocyte interferon-inducible peptide, NEDD5 and Wnt gene family members – Wnt5A, SARP1/hFRP-2, dishevelled and JNK2. Most of them have not been previously associated with pancreatic malignancy.
Golgi sialoglycoprotein MG-160, GLG1 (also called cysteine-rich fibroblast growth factor receptor) was the gene that displayed the highest differential in the analysis of purified tumour cells compared to normal pancreas (more than 10-fold upregulation). Its overexpression was additionally confirmed by quantitative real-time RT–PCR (Figure 4). Its postulated involvement in mediating secretion of fibroblast growth factors makes it a plausible candidate given the important role of this family in pancreatic tumorigenesis (Kohl et al., 2000).
NEDD5 protein homologue KIAA0158 is a cytoskeletal GTPase involved in cytokinesis and other cellular processes (Kinoshita et al., 1997). It was found consistently upregulated in all our experiments and it would be interesting to investigate further the role of this gene in the process of tumorigenesis.
Several members of the Wnt5A gene family appeared deregulated in pancreatic cancers, namely secreted apoptosis-related protein 1 (SARP1)/hFRP-2, dishevelled, JNK2 and Wnt5A itself, implicating the importance of this pathway in pancreatic cancer growth. In addition, SKY (spectral karyotype analysis) data from our laboratory show that deletion of the chromosomal region containing Wnt5A (13p14–21) in six out of 20 (30%) pancreatic carcinoma cell lines, while the SARP1/hFRP-2 locus (4q) is deleted in eight out of 20 cell lines (40%) (Sirivatanauksorn et al., 2001). Involvement of the Wnt5A gene family has not been previously associated with pancreatic adenocarcinoma. Wnt5A was described as a tumour suppressor gene in uroepithelial malignancies, and aberrant expression was found in lung, breast and prostate carcinomas as well as in melanomas (Iozzo et al., 1995; Olson et al., 1997). SARP-1/hFRP-2 belongs to a family of secreted glycoproteins that modulate the signalling activity of Wnt proteins. It is highly expressed in normal pancreas (Hu et al., 1998). Up-regulation of SARP-1/hFRP-2 is associated with growth inhibition, and down-regulation might contribute to malignancy. Lower expression of this gene was found in five out of five breast carcinomas, two out of four ovary tumours and three out of five kidney carcinomas (Zhou et al., 1998). In our experiments, it was downregulated sevenfold relative to normal pancreas (Table 2). Dysregulated expression of dishevelled 1 and c-jun N-terminal kinase 2, which are components of the same pathway, was also observed and warrants further investigation of Wnt signalling in pancreatic tumorigenesis.
The results described demonstrate the practical value of cDNA array technology in deciphering the molecular genetics of pancreatic adenocarcinoma. The gene expression profiles of predominantly stromal elements and epithelial tumour components isolated by cell aspiration give an insight into molecular cross-talk between tumour cells and their microenvironment that contributes to the biological behaviour of pancreatic cancer. Although the study was performed on a limited number of samples, it has nevertheless highlighted several genes that are potential candidates for further investigation and may prove useful for improving diagnosis and therapy of this highly malignant tumour.
Materials and methods
Tissue specimens, aspirates and cell lines
Pancreatectomy specimens were obtained at the Department of Pathology, University of Verona, Italy with full ethical approval from the host institution.
Snap-frozen samples from six pancreatic ductal adenocarcinomas prepared immediately following surgical resection were used to characterize gene expression profiles in bulk neoplastic tissue. Cryostat sections were analysed to verify the neoplastic cellularity, which was typically 30–40%.
Three of those six bulk specimens were also used as a source for fine needle aspirations. Immediately after the surgery, the freshly resected tumour mass was aspirated using a sterile syringe with a 21G needle to obtain enriched populations of neoplastic cells. A total of five aspirations were made in each case; they were pooled and quickly rinsed with phosphate-buffered saline. A small aliquot was used for cytological and immunocytochemical examination for epithelial cytokeratins (anticytokeratin 8/18 and CAM5.2 [anticytokeratin 7/8]; both from Research Monoclonal Antibodies, ICRF Clare Hall, Middlesex, UK). Only aspirates that contained more than 95% tumour cells were used in the study. The remainder of the aspirated material was processed immediately for RNA extraction.
Real-time quantitative RT–PCR was performed on a total of 20 adenocarcinoma cases, including the six used for the array studies (Tm15–Tm20) and six pancreatic cell lines. Mia PaCa-2, PANC-1 and BxPC-3 were obtained from the American Type Culture Collection (Rockville, MD, USA; PaTu 8988s and PaTu 8988t from Dr HF Kern (Department of Cell Biology, Philipps University, Marburg, Germany) and HPAF from Dr R Metzger, (University of N. Carolina, Durham, USA). All cell lines were cultured in RPMI-1640 medium (ICRF Media Production, Clare Hall, Middlesex, UK) supplemented with 10% heat-inactivated foetal calf serum (Gibco-BRL, Life Technologies, Paisley, UK).
As the non-neoplastic areas of the pancreas in cancer cases are usually affected by chronic pancreatitis, a snap-frozen, normal pancreas not suitable for transplantation from an organ donor was used as the control in all phases of the study. A normal pancreatic RNA sample purchased from Clontech (Basingstoke, Hampshire, UK) was used as an additional control in real time RT–PCR experiments.
RNA preparation
Total RNA from tissue specimens, cellular aspirates and cell lines was prepared using TRIZOL reagent (Gibco-BRL, Life Technologies Inc., Frederick, MA, USA) according to the manufacturer's protocol. The yield from the cellular aspirates, containing around 1–2×106 cells, was typically 2–5 μg. The quality of RNA from each sample was verified by electrophoresis through agarose gel and visualization with SYBR Gold dye (Molecular Bioprobes, Eugene, Oregon, USA).
Probe labelling and hybridization
The profiles of gene expression were investigated using Human Cancer Atlas cDNA Expression Array membranes (Clontech, Palo Alto, CA, USA). The arrays comprise sequences of 588 annotated cancer-associated genes belonging to various functional groups and include negative control and housekeeping genes (http:/www.clontech.com/atlas/genelists/index.shtml).
To reduce the effect of biological heterogeneity of tumour samples in our studies, and obtain the profile of genes commonly expressed in pancreatic adenocarcinomas, each hybridization was performed with a pool of three samples. Three pairs of filters were used. One filter from each pair was hybridized with control cDNA prepared from the normal organ donor pancreas. Two filters (from filter pair 1 and 2) were hybridized with target cDNA prepared from two different sets of three pooled adenocarcinomas, and the third one (from filter pair 3) was probed with the cDNA synthesized from three pooled tumour aspirates.
The membranes were hybridized according to the manufacturer's specifications. In each case, an aliquot of 2 μg of total RNA was reverse transcribed with [α-32P]dATP (10 mCi/ml, 3000 Ci/mmol; Amersham Pharmacia Biotech, UK) and the reagents supplied in the Atlas kit to synthesize 32P-labelled cDNA target. The membranes were hybridized overnight with 1.5×106 c.p.m./ml of ExpressHyb hybridization solution (Clontech, Palo Alto, CA, USA) at 68°C in a Hybaid roller bottle incubator. Membranes were washed twice with 2×SSC/1% and twice with 0.1×SSC/0.5% SDS at 68°C for 30 min each. Filters were exposed for up to 3 days in phosphor storage cassettes and scanned in a phosphorimager (Molecular Dynamics, Sunnyvale, CA, USA).
Data analysis
Image analysis and quantitation was performed with AtlasImage 1.01a software (Clontech Laboratories, Palo Alto, CA, USA). After grid assignment, the intensity value was calculated for each gene; as the arrays used were double-spotted, intensity was calculated as the average of the total signal (pixel value obtained after phosphorimager scanning) from the left and right spots. After the subtraction of background, global normalization was performed in the following way: the normalization coefficient was calculated by dividing the average values of all the genes from Array-1 (normal) with Array-2 (cancer) which was then used to multiply the intensity value of each gene. That gives the Adjusted Intensity. The ratio for each gene is then calculated by dividing the Array-2 Adjusted Intensity by the Adjusted Intensity of Array-1. A gene was considered to be expressed if the Intensity was more than twice the background level, and a ratio of twofold (0.5) was taken as the cut-off value for scoring upregulated (or downregulated) expression. When the Adjusted Intensity of a particular gene was less than twice the background on one of the arrays, the ratio could not be calculated as it would mean dividing by ‘0' (‘Nc’ in Table 2), therefore the Difference in Intensity values was calculated instead (Difference = Array-2 Adjusted Intensity – Array-1 Adjusted Intensity). A Difference with a negative value represents a gene downregulated in cancer.
As AtlasImage is designed for comparison of two arrays from each individual experiment and not for multifilter comparisons, the data obtained from different experiments were analysed in more detail using the GeneSpring program (Silicon Genetics). The 588 genes represented on the Atlas array were entered as a new ‘genome’, and subclassified into functional groups according to the list from Clontech (http://www.clontech.com/atlas/genelists/index.shtml). All expressed genes (intensities more than twice the background) from individual experiments were compared and Venn diagrams created to enable us to visualise the intersections (commonly expressed genes) between different experiments. The full lists of gene expression profiles from both tumour and normal pancreas created in this way are availableas supplementary data (http://www.lif.icnet.uk/axp/mphh/Supplementary/index.html).
Real-time quantitative RT–PCR
Real-time quantitative RT–PCR was used as an independent method to confirm up-regulation of two differentially expressed genes. Quantitative measurements of Rac 1 and GLG1 expression were obtained in 20 pancreatic cancer tissues, two matched non-neoplastic samples (coded as 4N and 10N), two normal pancreatic samples and six cell lines. cDNAs were synthesized from 1 to 5 μg total RNA using an oligo dT primer and the Superscript II reverse transcription kit (Gibco-BRL, Life Technologies, Paisley, Scotland). The cDNAs were column-purified (Qiagen, Crawley, West Sussex, UK) and eluted at a concentration of 10 ng/μl. The primers used for PCR were as follows: Rac 1 sense, 5′-AG CCTTCGCACTCAATGCCAAC-3′; Rac 1 antisense, 5′-TCGGCACAACAATGCAGTG TAG-3′; GLG1 sense, 5′-CGCTCTTCTCAGAAGTGACACC-3′; GLG1 antisense, 5′-GAGAGACTTGTCTACAGGCAGG-3′. These amplify a 249 and 190 bp fragment from the 3′ untranslated region of the Rac 1 and GLG1 transcripts respectively. Reactions containing 10 ng cDNA, SYBR green sequence detection reagents (Applied Biosystems) and sense and antisense primers were assayed on an ABI7700 sequence detection system (Applied Biosystems). The PCR conditions were 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 10 min, and 40 cycles of 95°C for 15 s, 65°C for 1 min. The accumulation of PCR product was measured in real time as the increase in SYBR green fluorescence, and the data were analysed using the Sequence Detector program v1.6.3 (Applied Biosystems). Standard curves relating initial template copy number to fluorescence and amplification cycle were generated using the amplified PCR product as a template, and were used to calculate Rac 1 (or GLG1) copy number in each sample.
References
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM . 2000 Nature 403: 503–511
Asakura S, Kato H, Fujino S, Konishi T, Tezuka N, Mori A . 1999 Hum. Pathol. 30: 195–198
Aspinall RJ, Lemoine NR . 1999 Ann. Oncol. 10: 188–192
Berditchevski F, Odintsova E . 1999 J. Cell Biol. 146: 477–492
Bramhall SR, Neoptolemos JP, Stamp GW, Lemoine NR . 1997 J. Pathol. 182: 347–355
Cardone A, Tolino A, Zarcone R, Borruto Caracciolo G, Tartaglia E . 1997 Panminerva Med. 39: 174–177
Chen FW, Davies JP, Ioannou YA . 1998 Mol. Genet. Metab. 64: 271–282
Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA . 1996 Science 274: 988–1001
Fritz G, Just I, Kaina B . 1999 Int. J. Cancer 81: 682–687
Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, Bloomfield CD, Lander ES . 1999 Science 286: 531–537
Gress TM, Menke A, Bachem M, Muller-Pillasch F, Ellenrieder V, Weidenbach H, Wagner M, Adler G . 1998 Digestion 59: 625–637
Gress TM, Muller-Pillasch F, Geng M, Zimmerhackl F, Zehetner G, Friess H, Buchler M, Adler G, Lehrach H . 1996 Oncogene 13: 1819–1830
Gress TM, Muller-Pillasch F, Lerch MM, Friess H, Buchler M, Adler G . 1995 Int. J. Cancer 62: 407–413
Gress TM, Wallrapp C, Frohme M, Muller-Pillasch F, Lacher U, Friess H, Buchler M, Adler G, Hoheisel JD . 1997 Genes Chromosomes Cancer 19: 97–103
Halvorsen TB, Seim E . 1989 J. Clin. Pathol. 42: 162–166
Hu E, Zhu Y, Fredrickson T, Barnes M, Kelsell D, Beeley L, Brooks D . 1998 Biochem. Biophys. Res. Commun. 247: 287–293
Iozzo RV, Eichstetter I, Danielson KG . 1995 Cancer Res. 55: 3495–3499
Kinoshita M, Kumar S, Mizoguchi A, Ide C, Kinoshita A, Haraguchi T, Hiraoka Y, Noda M . 1997 Genes Dev. 11: 1535–1547
Kohl R, Antoine M, Olwin BB, Dickson C, Kiefer P . 2000 J. Biol. Chem. 275: 15741–15748
Kornmann M, Kleeff J, Debinski W, Korc M . 1999 Anticancer Res. 19: 125–131
Lemoine NR . 1997 Digestion 58: 550–556
Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H, Jesnowski R . 2001 Cancer Res. 61: 550–555
Moscatello D, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV . 1998 J. Clin. Invest. 101: 406–412
Olson DJ, Gibo DM, Saggers G, Debinski W, Kumar R . 1997 Cell Growth Differ. 8: 417–423
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D . 2000 Nature 406: 747–752
Qiu RG, Chen J, Kirn D, McCormick F, Symons M . 1995 Nature 374: 457–459
Ricciardelli C, Mayne K, Sykes PJ, Raymond WA, McCaul K, Marshall VR, Horsfall D . 1998 Clin. Cancer Res. 4: 963–971
Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, Pergamenschikov A, Lee JC, Lashkari D, Shalon D, Myers TG, Weinstein JN, Botstein D, Brown PO . 2000 Nat. Genet. 24: 227–235
Ryu B, Jones J, Hollingsworth MA, Hruban RH, Kern SE . 2001 Cancer Res. 61: 1833–1838
Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E . 2000 Oncogene 19: 3013–3020
Sho M, Adachi M, Taki T, Hashida H, Konishi T, Huang CL, Ikeda N, Nakajima Y, Kanehiro H, Hisanaga M, Nakano H, Miyake M . 1998 Int. J. Cancer 79: 509–516
Shoemaker DD, Schadt EE, Armour CD, He YD, Garrett-Engele P, McDonagh PD, Loerch PM, Leonardson A, Lum PY, Cavet G, Wu LF, Altschuler SJ, Edwards S, King J, Tsang JS, Schimmack G, Schelter JM, Koch J, Ziman M, Marton MJ, Li B, Cundiff P, Ward T, Castle J, Krolewski M, Meyer MR, Mao M, Burchard J, Kidd MJ, Dai H, Phillips JW, Linsley PS, Stoughton R, Scherer S, Boguski MS . 2001 Nature 409: 922–927
Sirivatanauksorn V, Sirivatanauksorn Y, Gorman PA, Davidson JM, Sheer D, Moore PS, Scarpa A, Edwards PA, Lemoine NR . 2001 Int. J. Cancer 91: 350–358
Sirivatanauksorn Y, Drury R, Crnogorac-Jurcevic T, Sirivatanauksorn V, Lemoine NR . 1999 J. Pathol. 189: 150–154
Suwa H, Ohshio G, Imamura T, Watanabe G, Arii S, Imamura M, Narumiya S, Hiai H, Fukumoto M . 1998 Br. J. Cancer 77: 147–152
Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y . 1997 J. Cell Biol. 139: 1047–1059
Virkajarvi N, Paakko P, Soini Y . 1998 Histopathology 33: 432–439
Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW . 1997 Science 276: 1268–1272
Zhou Z, Wang J, Han X, Zhou J, Linder S . 1998 Int. J. Cancer 78: 95–99
Acknowledgements
Supported by Imperial Cancer Research Fund; Special Trustees of Hammersmith Hospital; Associazione Italiana Ricerca sul Cancro (AIRC) to A Scarpa, Milan, Italy; Consorzio Studi Universitari di Verona, Italy; Ministero Università e Ricerca Scientifica e Tecnologica (MURST), Cofin MM06158571 – Cofin 9906195987, Rome, Italy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crnogorac-Jurcevic, T., Efthimiou, E., Capelli, P. et al. Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene 20, 7437–7446 (2001). https://doi.org/10.1038/sj.onc.1204935
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.onc.1204935
Keywords
This article is cited by
-
Rac1 activation in oral squamous cell carcinoma as a predictive factor associated with lymph node metastasis
International Journal of Clinical Oncology (2023)
-
Circular RNA hsa_circ_0078607 suppresses ovarian cancer progression by regulating miR-518a-5p/Fas signaling pathway
Journal of Ovarian Research (2020)
-
MicroRNAs in Pancreatic Cancer: biomarkers, prognostic, and therapeutic modulators
BMC Cancer (2019)
-
Targeting acquired oncogenic burden in resilient pancreatic cancer: a novel benefit from marine polyphenols
Molecular and Cellular Biochemistry (2019)
-
The hepatic pre-metastatic niche in pancreatic ductal adenocarcinoma
Molecular Cancer (2018)