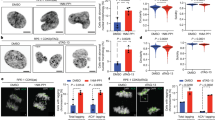
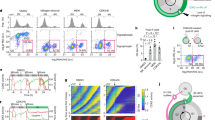
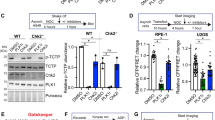
Abstract
Protein kinases play a pivotal role in execution of cell division. Polo and Polo-like kinases have emerged as major regulators for various cell cycle checkpoints. Early genetic studies have demonstrated that CDC5, a budding yeast counterpart of vertebrate Plks, is essential for successful mitotic progression. Mammalian Plks localize primarily to the centrosome during interphase and the mitotic apparatus during mitosis. Many key cell cycle regulators such as p53, Cdc25C, cyclin B, components of the anaphase-promoting complex, and mitotic motor proteins are directly targeted by Plks. Although the exact mechanism of action of these protein kinases in vivo remains to be elucidated, Plks are important mediators for various cell cycle checkpoints that monitor centrosome duplication, DNA replication, formation of bipolar mitotic spindle, segregation of chromosomes, and mitotic exit, thus protecting cells against genetic instability during cell division.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Abrieu A, Brassac T, Galas S, Fisher D, Labbe JC and Doree M . (1998). J. Cell Sci., 111 (Part 12), 1751–1757.
Adams RR, Tavares AA, Salzberg A, Bellen HJ and Glover DM . (1998). Genes Dev., 12, 1483–1494.
Ahn JY, Schwarz JK, Piwnica-Worms H and Canman CE . (2000). Cancer Res., 60, 5934–5936.
Alexandru G, Uhlmann F, Mechtler K, Poupart MA and Nasmyth K . (2001). Cell, 105, 459–472.
Ando K, Ozaki T, Yamamoto H, Furuya K, Hosoda M, Hayashi S, Fukuzawa M and Nakagawara A . (2004). J. Biol. Chem., 279, 25549–25561.
Arnaud L, Pines J and Nigg EA . (1998). Chromosoma, 107, 424–429.
Bahassi EM, Conn CW, Myer DL, Hennigan RF, McGowan CH, Sanchez Y and Stambrook PJ . (2002). Oncogene, 21, 6633–6640.
Bahassi EM, Hennigan RF, Myer DL and Stambrook PJ . (2004). Oncogene, 23, 2658–2663.
Bahler J, Steever AB, Wheatley S, Wang Y, Pringle JR, Gould KL and McCollum D . (1998). J. Cell Biol., 143, 1603–1616.
Barr FA, Sillje HH and Nigg EA . (2004). Nat. Rev. Mol. Cell Biol., 5, 429–440.
Bartholomew CR, Woo SH, Chung YS, Jones C and Hardy CF . (2001). Mol. Cell. Biol., 21, 4949–4959.
Bharadwaj R and Yu H . (2004). Oncogene, 23, 2016–2027.
Brassac T, Castro A, Lorca T, Le Peuch C, Doree M, Labbe JC and Galas S . (2000). Oncogene, 19, 3782–3790.
Budde PP, Kumagai A, Dunphy WG and Heald R . (2001). J. Cell Biol., 153, 149–158.
Burns TF, Fei P, Scata KA, Dicker DT and El Deiry WS . (2003). Mol. Cell. Biol., 23, 5556–5571.
Carmena M, Riparbelli MG, Minestrini G, Tavares AM, Adams R, Callaini G and Glover DM . (1998). J. Cell Biol., 143, 659–671.
Casenghi M, Meraldi P, Weinhart U, Duncan PI, Korner R and Nigg EA . (2003). Dev. Cell, 5, 113–125.
Cheng L, Hunke L and Hardy CF . (1998). Mol. Cell. Biol., 18, 7360–7370.
Conn CW, Hennigan RF, Dai W, Sanchez Y and Stambrook PJ . (2000). Cancer Res., 60, 6826–6831.
Dai W and Cogswell JP . (2003). Prog. Cell Cycle Res., 5, 327–334.
Dai W, Wang Q and Traganos F . (2002). Oncogene, 21, 6195–6200.
Deming PB, Flores KG, Downes CS, Paules RS and Kaufmann WK . (2002). J. Biol. Chem., 277, 36832–36838.
Elledge SJ . (1996). Science, 274, 1664–1672.
Feng Y, Longo DL and Ferris DK . (2001). Cell Growth Differ., 12, 29–37.
Golan A, Yudkovsky Y and Hershko A . (2002). J. Biol. Chem., 277, 15552–15557.
Golsteyn RM, Schultz SJ, Bartek J, Ziemiecki A, Ried T and Nigg EA . (1994). J. Cell Sci., 107 (Part 6), 1509–1517.
Hamanaka R, Maloid S, Smith MR, O'Connell CD, Longo DL and Ferris DK . (1994). Cell Growth Differ., 5, 249–257.
Haracska L, Prakash S and Prakash L . (2003). Mol. Cell. Biol., 23, 1453–1459.
Heitz MJ, Petersen J, Valovin S and Hagan IM . (2001). J. Cell Sci., 114, 4521–4532.
Hubscher U, Maga G and Spadari S . (2002). Annu. Rev. Biochem., 71, 133–163.
Hudson JW, Kozarova A, Cheung P, Macmillan JC, Swallow CJ, Cross JC and Dennis JW . (2001). Curr. Biol., 11, 441–446.
Hu F, Wang Y, Liu D, Li Y, Qin J and Elledge SJ . (2001). Cell, 107, 655–665.
Jackman M, Lindon C, Nigg EA and Pines J . (2003). Nat. Cell Biol., 5, 143–148.
Kang D, Chen J, Wong J and Fang G . (2002). J. Cell Biol., 156, 249–260.
Kotani S, Tugendreich S, Fujii M, Jorgensen PM, Watanabe N, Hoog C, Hieter P and Todokoro K . (1998). Mol. Cell, 1, 371–380.
Lane HA and Nigg EA . (1996). J. Cell Biol., 135, 1701–1713.
Lee BH and Amon A . (2003). Science, 300, 482–486.
Lee KS and Erikson RL . (1997). Mol. Cell. Biol., 17, 3408–3417.
Li B, Ouyang B, Pan H, Reissmann PT, Slamon DJ, Arceci R, Lu L and Dai W . (1996). J. Biol. Chem., 271, 19402–19408.
Lin CY, Madsen ML, Yarm FR, Jang YJ, Liu X and Erikson RL . (2000). Proc. Natl. Acad. Sci. USA, 97, 12589–12594.
Lindon C and Pines J . (2004). J. Cell Biol., 164, 233–241.
Litvak V, Argov R, Dahan N, Ramachandran S, Amarilio R, Shainskaya A and Lev S . (2004). Mol. Cell, 14, 319–330.
Litvak V, Tian D, Carmon S and Lev S . (2002). Mol. Cell. Biol., 22, 5064–5075.
Liu X and Erikson RL . (2002). Proc. Natl. Acad. Sci. USA, 99, 8672–8676.
Losada A, Hirano M and Hirano T . (2002). Genes Dev., 16, 3004–3016.
Moshe Y, Boulaire J, Pagano M and Hershko A . (2004). Proc. Natl. Acad. Sci. USA, 101, 7937–7942.
Mulvihill DP and Hyams JS . (2002). J. Cell Sci., 115, 3575–3586.
Musacchio A and Hardwick KG . (2002). Nat. Rev. Mol. Cell Biol., 3, 731–741.
Neef R, Preisinger C, Sutcliffe J, Kopajtich R, Nigg EA, Mayer TU and Barr FA . (2003). J. Cell Biol., 162, 863–875.
Nigg EA . (1998). Cell Biol., 10, 776–783.
Ohkura H, Hagan IM and Glover DM . (1995). Genes Dev., 9, 1059–1073.
Ouyang B, Li W, Pan H, Meadows J, Hoffmann I and Dai W . (1999). Oncogene, 18, 6029–6036.
Ouyang B, Pan H, Lu L, Li J, Stambrook P, Li B and Dai W . (1997). J. Biol. Chem., 272, 28646–28651.
Park CJ, Song S, Giddings Jr TH, Ro HS, Sakchaisri K, Park JE, Seong YS, Winey M and Lee KS . (2004). Mol. Biol. Cell, 15, 1711–1723.
Qian YW, Erikson E, Taieb FE and Maller JL . (2001). Mol. Biol. Cell, 12, 1791–1799.
Roshak AK, Capper EA, Imburgia C, Fornwald J, Scott G and Marshall LA . (2000). Cell Signal., 12, 405–411.
Ruan Q, Wang Q, Xie S, Fang Y, Darzynkiewicz Z, Guan K, Jhanwar-Uniyal M and Dai W . (2004). Exp. Cell Res., 294, 51–59.
Sancar A, Lindsey-Boltz LA, Unsal-Kaccmaz K and Linn S . (2004). Annu. Rev. Biochem., 73, 39–85.
Sanchez Y, Bachant J, Wang H, Hu F, Liu D, Tetzlaff M and Elledge SJ . (1999). Science, 286, 1166–1171.
Schwab M, Neutzner M, Mocker D and Seufert W . (2001). EMBO J., 20, 5165–5175.
Seong YS, Kamijo K, Lee JS, Fernandez E, Kuriyama R, Miki T and Lee KS . (2002). J. Biol. Chem., 277, 32282–32293.
Shimizu-Yoshida Y, Sugiyama K, Rogounovitch T, Ohtsuru A, Namba H, Saenko V and Yamashita S . (2001). Biochem. Biophys. Res. Commun., 289, 491–498.
Shtivelman E . (2003). Mol. Cancer Res., 1, 959–969.
Simmons DL, Neel BG, Stevens R, Evett G and Erikson RL . (1992). Mol. Cell. Biol., 12, 4164–4169.
Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA and Medema RH . (2000). Nat. Cell Biol., 2, 672–676.
Stegmeier F, Visintin R and Amon A . (2002). Cell, 108, 207–220.
Sunkel CE and Glover DM . (1988). J. Cell Sci., 89 (Part 1), 25–38.
Toczyski DP, Galgoczy DJ and Hartwell LH . (1997). Cell, 90, 1097–1106.
Toyoshima-Morimoto F, Taniguchi E and Nishida E . (2002). EMBO Rep., 3, 341–348.
Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A and Nishida E . (2001). Nature, 410, 215–220.
Tsvetkov L, Xu X, Li J and Stern DF . (2003). J. Biol. Chem., 278, 8468–8475.
van Vugt MA, Smits VA, Klompmaker R and Medema RH . (2001). J. Biol. Chem., 276, 41656–41660.
van Vugt MA, Van De Weerdt BC, Vader G, Janssen H, Calafat J, Klompmaker R, Wolthuis RM and Medema RH . (2004). J. Biol. Chem., 279, 36841–36854.
Walsh S, Margolis SS and Kornbluth S . (2003). Mol. Cancer Res., 1, 280–289.
Wang Q, Xie S, Chen J, Fukasawa K, Naik U, Traganos F, Darzynkiewicz Z, Jhanwar-Uniyal M and Dai W . (2002). Mol. Cell. Biol., 22, 3450–3459.
Xie S-Q, Wu H-Y, Wang Q, Kunicki J, Thomas RO, Hollingsworth RE, Cogswell J and Dai W . (2002). Cell Cycle, 1, 424–429.
Xie S, Wang Q, Ruan Q, Liu T, Jhanwar-Uniyal M, Guan K and Dai W . (2004). Oncogene, 23, 3822–3829.
Xie S, Wang Q, Wu H, Cogswell J, Lu L, Jhanwar-Uniyal M and Dai W . (2001a). J. Biol. Chem., 276, 36194–36199.
Xie S, Wu H, Wang Q, Cogswell JP, Husain I, Conn C, Stambrook P, Jhanwar-Uniyal M and Dai W . (2001b). J. Biol. Chem., 276, 43305–43312.
Xu J and Du W . (2003). FEBS Lett., 545, 209–212.
Yarm FR . (2002). Mol. Cell. Biol., 22, 6209–6221.
Yoo HY, Kumagai A, Shevchenko A, Shevchenko A and Dunphy WG . (2004). Cell, 117, 575–588.
Yoshida S and Toh-e A . (2002). Biochem. Biophys. Res. Commun., 294, 687–691.
Yuan J, Eckerdt F, Bereiter-Hahn J, Kurunci-Csacsko E, Kaufmann M and Strebhardt K . (2002). Oncogene, 21, 8282–8292.
Zeng XR, Hao H, Jiang Y and Lee MY . (1994). J. Biol. Chem., 269, 24027–24033.
Zhou BB and Elledge SJ . (2000). Nature, 408, 433–439.
Zhou T, Aumais JP, Liu X, Yu-Lee LY and Erikson RL . (2003). Dev. Cell, 5, 127–138.
Acknowledgements
We apologize to many authors whose articles could not be cited in this review due to space limitations. We thank Dr Frank Traganos for critical reading of the manuscript and the members in Dr Dai's laboratory for helpful discussions. We also thank Ms Lisa Buerle for editorial and administrative assistance. The work is supported in part by grants from the National Institutes of Health to WD (CA90658 and CA74229) and to ML (GM31973).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, S., Xie, B., Lee, M. et al. Regulation of cell cycle checkpoints by polo-like kinases. Oncogene 24, 277–286 (2005). https://doi.org/10.1038/sj.onc.1208218
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.onc.1208218
Keywords
This article is cited by
-
Phase 1 dose escalation trial of volasertib in combination with decitabine in patients with acute myeloid leukemia
International Journal of Hematology (2021)
-
The RIO protein kinase-encoding gene Sj-riok-2 is involved in key reproductive processes in Schistosoma japonicum
Parasites & Vectors (2017)
-
Polo-like Kinase 1 as a potential therapeutic target in Diffuse Intrinsic Pontine Glioma
BMC Cancer (2016)
-
Putting a bit into the polo-box domain of polo-like kinase 1
Journal of Analytical Science and Technology (2015)
-
Abortive Cell Cycle Events in the Brains of Scrapie-Infected Hamsters with Remarkable Decreases of PLK3/Cdc25C and Increases of PLK1/Cyclin B1
Molecular Neurobiology (2013)