Abstract
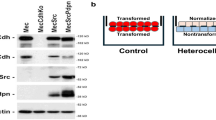
Anchorage-independent growth is a hallmark of tumor growth and results from enhanced proliferation and altered cell–cell and cell-matrix interactions. By using gene-deficient mouse embryonic fibroblasts (MEFs), we showed for the first time that NHERF1/EBP50 (Na/H exchanger regulator factor 1/ezrin-radixin-moesin binding phosphoprotein 50), an adapter protein with membrane localization under physiological conditions, inhibits cell motility and is required to suppress anchorage-independent growth. Both NHERF1 PDZ domains are necessary for the tumor suppressor effect. NHERF1 associates directly through the PDZ2 domain with β-catenin and is required for β-catenin localization at the cell–cell junctions in MEFs. Mechanistically, the absence of NHERF1 selectively decreased the interaction of β-catenin with E-cadherin, but not with N-cadherin. The ensuing disorganization of E-cadherin-mediated adherens junctions as well as the observed moderate increase in β-catenin transcriptional activity contributed most likely to the anchorage-independent growth of NHERF1-deficient MEFs. In vivo, NHERF1 is specifically localized at the apical brush-border membrane in intestinal epithelial cells and is required to maintain a fraction of the cortical β-catenin at this level. Thus, NHERF1 emerges as a cofactor essential for the integrity of epithelial tissues by maintaining the proper localization and complex assembly of β-catenin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W . (2004). Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev 18: 2225–2230.
Chen YT, Stewart DB, Nelson WJ . (1999). Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Biol 144: 687–699.
Dai JL, Wang L, Sahin AA, Broemeling LD, Schutte M, Pan Y . (2004). NHERF (Na+/H+ exchanger regulatory factor) gene mutations in human breast cancer. Oncogene 23: 8681–8687.
Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H . (1999). The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA 96: 10182–10187.
Giles RH, van Es JH, Clevers H . (2003). Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653: 1–24.
Gottardi CJ, Gumbiner BM . (2004). Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol 167: 339–349.
McCrea PD, Gumbiner BM . (1991). Purification of a 92-kDa cytoplasmic protein tightly associated with the cell-cell adhesion molecule E-cadherin (uvomorulin). Characterization and extractability of the protein complex from the cell cytostructure. J Biol Chem 266: 4514–4520.
Morales FC, Takahashi Y, Kreimann EL, Georgescu M-M . (2004). Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci USA 101: 17705–17710.
Nelson WJ, Nusse R . (2004). Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303: 1483–1487.
Provost E, Rimm DL . (1999). Controversies at the cytoplasmic face of the cadherin-based adhesion complex. Curr Opin Cell Biol 11: 567–572.
Reczek D, Berryman M, Bretscher A . (1997). Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol 139: 169–179.
Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z . (1994). Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol 14: 8333–8342.
Sadot E, Simcha I, Shtutman M, Ben-Ze'ev A, Geiger B . (1998). Inhibition of beta-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci USA 95: 15339–15344.
Shenolikar S, Voltz JW, Cunningham R, Weinman EJ . (2004). Regulation of ion transport by the NHERF family of PDZ proteins. Physiology (Bethesda) 19: 362–369.
Shibata T, Chuma M, Kokubu A, Sakamoto M, Hirohashi S . (2003). EBP50, a beta-catenin-associating protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology 38: 178–186.
Suzuki J, Sukezane T, Akagi T, Georgescu MM, Ohtani M, Inoue H et al. (2004). Loss of c-abl facilitates anchorage-independent growth of p53- and RB-deficient primary mouse embryonic fibroblasts. Oncogene 23: 8527–8534.
Takahashi Y, Morales FC, Kreimann EL, Georgescu MM . (2006). PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBO J 25: 910–920.
Takeichi M . (1995). Morphogenetic roles of classic cadherins. Curr Opin Cell Biol 7: 619–627.
Thiery JP . (2002). Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2: 442–454.
Voltz JW, Weinman EJ, Shenolikar S . (2001). Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene 20: 6309–6314.
Weinman EJ, Wang Y, Wang F, Greer C, Steplock D, Shenolikar S . (2003). A C-terminal PDZ motif in NHE3 binds NHERF-1 and enhances cAMP inhibition of sodium-hydrogen exchange. Biochemistry 42: 12662–12668.
Acknowledgements
MMG acknowledges support from MD Anderson Cancer Center Tobacco Fund and NCI-CA107201, FCM from American Brain Tumor Association and ELK from NCI-CA09299-26. NCI-CA16672 partially supported animal breeding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Kreimann, E., Morales, F., de Orbeta-Cruz, J. et al. Cortical stabilization of β-catenin contributes to NHERF1/EBP50 tumor suppressor function. Oncogene 26, 5290–5299 (2007). https://doi.org/10.1038/sj.onc.1210336
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.onc.1210336
Keywords
This article is cited by
-
Ezrin–Radixin–Moesin Binding Phosphoprotein 50: A Potential Novel Biomarker in Human Papilloma Virus-Associated Head and Neck Squamous Cell Carcinomas
Head and Neck Pathology (2019)
-
NHERF1 and tumor microenvironment: a new scene in invasive breast carcinoma
Journal of Experimental & Clinical Cancer Research (2018)
-
Dynamic relocalization of NHERF1 mediates chemotactic migration of ovarian cancer cells toward lysophosphatidic acid stimulation
Experimental & Molecular Medicine (2017)
-
Role of the PDZ-scaffold protein NHERF1/EBP50 in cancer biology: from signaling regulation to clinical relevance
Oncogene (2017)
-
NHERF1/EBP50 and NF2 as diagnostic markers for choroid plexus tumors
Acta Neuropathologica Communications (2016)