Abstract
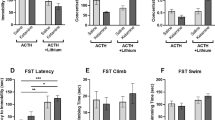
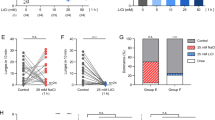
Lithium inhibits glycogen synthase kinase-3 (GSK-3) at therapeutic concentrations; however, it is unclear if this inhibition and its downstream effects on specific signaling pathways are relevant to the treatment of bipolar disorder and depression. One of the targets of GSK-3 is the transcription factor β-catenin. Normally active GSK-3 phosphorylates β-catenin, leading to its degradation. Inhibition of GSK-3 therefore increases β-catenin. We have utilized transgenic mice to investigate the behavioral consequences of CNS β-catenin overexpression. Transgenic mice overexpressing β-catenin demonstrated behavioral changes similar to those observed following the administration of lithium, including decreased immobility time in the forced swim test (FST). Further, we show that although acute administration of lithium and overexpression of the β-catenin transgene inhibits d-amphetamine-induced hyperlocomotion, neither lithium nor the β-catenin transgene prevents d-amphetamine-induced sensitization, as measured by locomotor activity. Both lithium-treated and β-catenin mice had an elevated response to d-amphetamine following multiple administrations of the stimulant, though the difference in absolute locomotion was maintained throughout the sensitization time-course. Neither acute lithium nor β-catenin overexpression had an effect on d-amphetamine-induced stereotyped behavior. The results of this study, in which β-catenin transgenic mice exhibited behaviors identical to those observed in lithium-treated mice, are consistent with the hypothesis that the behavioral effects of lithium in these models are mediated through its direct inhibition of GSK-3 and the consequent increase in β-catenin. By associating the behavioral effects of lithium with β-catenin levels, these data suggest that increasing β-catenin might be a novel therapeutic strategy for mood disorders.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aberle H, Bauer A, Stappert J, Kispert A, Kemler R (1997). Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16: 3797–3804.
Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR et al (2004). Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA 101: 5099–5104.
Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R et al (1998). Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 280: 596–599.
Benedetti F, Serretti A, Pontiggia A, Bernasconi A, Lorenzi C, Colombo C et al (2005). Long-term response to lithium salts in bipolar illness is influenced by the glycogen synthase kinase 3-beta-50 T/C SNP. Neurosci Lett 376: 51–55.
Borchelt DR, Davis J, Fischer M, Lee MK, Slunt HH, Ratovitsky T et al (1996). A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal 13: 159–163.
Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254.
Cappuccio I, Calderone A, Busceti CL, Biagioni F, Pontarelli F, Bruno V et al (2005). Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J Neurosci 25: 2647–2657.
Chenn A, Walsh CA (2002). Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297: 365–369.
Cohen P, Goedert M (2004). GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov 3: 479–487.
Cox C, Harrison-Read PE, Steinberg H, Tomkiewicz M (1971). Lithium attenuates drug-induced hyperactivity in rats. Nature 232: 336–338.
Crawley JN (2000). What's Wrong with My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice. Wiley-Liss: New York. xiii, 329pp.
Davies C, Sanger DJ, Steinberg H, Tomkiewicz M, U’Prichard DC (1974). Lithium and alpha-methyl-p-tyrosine prevent ‘manic’ activity in rodents. Psychopharmacologia 36: 263–274.
De Ferrari GV, Chacon MA, Barria MI, Garrido JL, Godoy JA, Olivares G et al (2003). Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol Psychiatry 8: 195–208.
Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L et al (2003). The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci 23: 7311–7316.
Fessler RG, Sturgeon RD, London SF, Meltzer HY (1982). Effects of lithium on behaviour induced by phencyclidine and amphetamine in rats. Psychopharmacology (Berlin) 78: 373–376.
Flemenbaum A (1977). Lithium inhibition of norepinephrine and dopamine receptors. Biol Psychiatry 12: 563–572.
Frances H, Marion MH, Simon P (1981). Antidepressant action of lithium: proposed mechanism based upon behavioral analysis in the mouse. Prog Neuropsychopharmacol 5: 357–361.
Furlong MT, Morin PJ (2000). Rare activation of the TCF/beta-catenin pathway in ovarian cancer. Gynecol Oncol 77: 97–104.
Gottesman II, Gould TD (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160: 636–645.
Gould TD, Chen G, Manji HK (2004a). In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology 29: 32–38.
Gould TD, Einat H, Bhat R, Manji HK (2004b). AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol 7: 387–390.
Gould TD, Gray NA, Manji HK (2003). Effects of a glycogen synthase kinase-3 inhibitor, lithium, in adenomatous polyposis coli mutant mice. Pharmacol Res 48: 49–53.
Gould TD, Manji HK (2005). Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology 30: 1223–1237.
Gould TD, O’Donnell KC, Picchini AM, Manji HK Strain difference in lithium attenuation of d-amphetamine hyperlocomotion: a mouse model for the genetics of lithium response. Neuropsychopharmacology [E-pub ahead of print].
Gould TD, Picchini AM, Einat H, Manji HK (2006). Targeting glycogen synthase kinase-3 in the CNS: implications for the development of new treatments for mood disorders. Curr Drug Targets 7: 1399–1409.
Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK (2004c). Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Mol Psychiatry 9: 734–755.
Hamburger-Bar R, Robert M, Newman M, Belmaker RH (1986). Interstrain correlation between behavioural effects of lithium and effects on cortical cyclic AMP. Pharmacol Biochem Behav 24: 9–13.
Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK (2006). Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry 60: 93–105.
Ilouz R, Kaidanovich O, Gurwitz D, Eldar-Finkelman H (2002). Inhibition of glycogen synthase kinase-3beta by bivalent zinc ions: insight into the insulin-mimetic action of zinc. Biochem Biophys Res Commun 295: 102–106.
Institute of Laboratory Animal Resources (US) (1996). Guide for the Care and Use of Laboratory Animals. National Academy Press: Washington, DC. xii, 125pp.
Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H (2004). Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry 55: 781–784.
Kelley AE (2005). Measurement of rodent stereotyped behavior. In: Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnick P, Wray S (eds). Current Protocols in Neuroscience. John Wiley & Sons: New York, NY. pp 8.8.1–8.8.13.
Klein PS, Melton DA (1996). A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA 93: 8455–8459.
Kratz JE, Stearns D, Huso DL, Slunt HH, Price DL, Borchelt DR et al (2002). Expression of stabilized beta-catenin in differentiated neurons of transgenic mice does not result in tumor formation. BMC Cancer 2: 33.
Kroczka B, Branski P, Palucha A, Pilc A, Nowak G (2001). Antidepressant-like properties of zinc in rodent forced swim test. Brain Res Bull 55: 297–300.
Kroczka B, Zieba A, Dudek D, Pilc A, Nowak G (2000). Zinc exhibits an antidepressant-like effect in the forced swimming test in mice. Pol J Pharmacol 52: 403–406.
Kwok JB, Hallupp M, Loy CT, Chan DK, Woo J, Mellick GD et al (2005). GSK3B polymorphisms alter transcription and splicing in Parkinson's disease. Ann Neurol 58: 829–839.
Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A et al (2005). Wnt signalling regulates adult hippocampal neurogenesis. Nature 437: 1370–1375.
Madsen TM, Newton SS, Eaton ME, Russell DS, Duman RS (2003). Chronic electroconvulsive seizure up-regulates beta-catenin expression in rat hippocampus: role in adult neurogenesis. Biol Psychiatry 54: 1006–1014.
Miyauchi T, Kikuchi K, Satoh S (1981). Further studies on the potentiating effect of lithium chloride on methamphetamine-induced stereotypy in mice. Jpn J Pharmacol 31: 61–68.
Naccarato WF, Ray RE, Wells WW (1974). Biosynthesis of myo-inositol in rat mammary gland. Isolation and properties of the enzymes. Arch Biochem Biophys 164: 194–201.
Namima M, Sugihara K, Watanabe Y, Sasa H, Umekage T, Okamoto K (1999). Quantitative analysis of the effects of lithium on the reverse tolerance and the c-Fos expression induced by methamphetamine in mice. Brain Res Brain Res Protoc 4: 11–18.
Nowak G, Szewczyk B, Wieronska JM, Branski P, Palucha A, Pilc A et al (2003). Antidepressant-like effects of acute and chronic treatment with zinc in forced swim test and olfactory bulbectomy model in rats. Brain Res Bull 61: 159–164.
O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S et al (2004). Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci 24: 6791–6798.
Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW (1997). Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem 272: 24735–24738.
Ozawa H, Miyauchi T (1977). Potentiating effect of lithium chloride on methamphetamine-induced stereotypy in mice. Eur J Pharmacol 41: 213–216.
Peifer M, Pai LM, Casey M (1994). Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Dev Biol 166: 543–556.
Porsolt RD, Le Pichon M, Jalfre M (1977). Depression: a new animal model sensitive to antidepressant treatments. Nature 266: 730–732.
Post RM, Weiss SR, Pert A (1984). Differential effects of carbamazepine and lithium on sensitization and kindling. Prog Neuropsychopharmacol Biol Psychiatry 8: 425–434.
Ray Jr WJ, Szymanki ES, Ng L (1978). The binding of lithium and of anionic metabolites to phosphoglucomutase. Biochim Biophys Acta 522: 434–442.
Rubin EH, Wooten GF (1984). The behavioral and biochemical effects of lithium on dopaminergic agonist-induced supersensitivity. Psychopharmacology (Berlin) 84: 217–220.
Stambolic V, Ruel L, Woodgett JR (1996). Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol 6: 1664–1668.
Wielosz M (1976). The effect of lithium chloride on the activity of some psychotropic drugs. Pol J Pharmacol Pharm 28: 189–198.
Yang P, Singhal N, Modi G, Swann A, Dafny N (2001). Effects of lithium chloride on induction and expression of methylphenidate sensitization. Eur J Pharmacol 426: 65–72.
York JD, Ponder JW, Majerus PW (1995). Definition of a metal-dependent/Li(+)-inhibited phosphomonoesterase protein family based upon a conserved three-dimensional core structure. Proc Natl Acad Sci USA 92: 5149–5153.
Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT (1996). The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 10: 1443–1454.
Acknowledgements
We thank Dr Charles Eberhart (Johns Hopkins University) for the β-catenin transgenic mice, Dr Francois Vautier for assistance with the Morris water maze protocol, and David A Luckenbaugh, NIMH Mood and Anxiety Disorders Program Biostatistician, for statistical assistance. This research was supported by the Intramural Research Program of the National Institute of Mental Heath, the Foundation for the National Institutes of Health (Neuroscience Research Fellowship (TDG)), the National Association for Research on Schizophrenia and Depression (Young Investigator Award to TDG and HE), and the Stanley Research Foundation (HKM). The authors have no conflicts of interest, financial or otherwise.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Rights and permissions
About this article
Cite this article
Gould, T., Einat, H., O'Donnell, K. et al. β-Catenin Overexpression in the Mouse Brain Phenocopies Lithium-Sensitive Behaviors. Neuropsychopharmacol 32, 2173–2183 (2007). https://doi.org/10.1038/sj.npp.1301338
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301338
Keywords
This article is cited by
-
Advances toward precision medicine for bipolar disorder: mechanisms & molecules
Molecular Psychiatry (2021)
-
Lithium: a potential therapeutic strategy in obsessive–compulsive disorder by targeting the canonical WNT/β pathway
Translational Psychiatry (2021)
-
The antidepressant roles of Wnt2 and Wnt3 in stress-induced depression-like behaviors
Translational Psychiatry (2016)
-
Genome-wide association study of bipolar disorder accounting for effect of body mass index identifies a new risk allele in TCF7L2
Molecular Psychiatry (2014)
-
β-catenin mediates stress resilience through Dicer1/microRNA regulation
Nature (2014)