Abstract
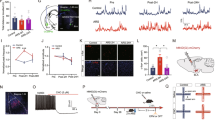
A wealth of research identifies the amygdala as a key brain region mediating negative affect, and implicates amygdala dysfunction in the pathophysiology of anxiety disorders. Although there is a strong genetic component to anxiety disorders such as posttraumatic stress disorder (PTSD) there remains debate about whether abnormalities in amygdala function predispose to these disorders. In the present study, groups of C57BL/6 × DBA/2 (B × D) recombinant inbred strains of mice were selected for differences in volume of the basolateral amygdala complex (BLA). Strains with relatively small, medium, or large BLA volumes were compared for Pavlovian fear learning and memory, anxiety-related behaviors, depression-related behavior, and glucocorticoid responses to stress. Strains with relatively small BLA exhibited stronger conditioned fear responses to both auditory tone and contextual stimuli, as compared to groups with larger BLA. The small BLA group also showed significantly greater corticosterone responses to stress than the larger BLA groups. BLA volume did not predict clear differences in measures of anxiety-like behavior or depression-related behavior, other than greater locomotor inhibition to novelty in strains with smaller BLA. Neither striatal, hippocampal nor cerebellar volumes correlated significantly with any behavioral measure. The present data demonstrate a phenotype of enhanced fear conditioning and exaggerated glucocorticoid responses to stress associated with small BLA volume. This profile is reminiscent of the increased fear processing and stress reactivity that is associated with amygdala excitability and reduced amygdala volume in humans carrying loss of function polymorphisms in the serotonin transporter and monoamine oxidase A genes. Our study provides a unique example of how natural variation in amygdala volume associates with specific fear- and stress-related phenotypes in rodents, and further supports the role of amygdala dysfunction in anxiety disorders such as PTSD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Airey DC, Lu L, Shou S, Williams RW (2002). Genetic sources of individual differences in the cerebellum. Cerebellum 1: 233–240.
Airey DC, Lu L, Williams RW (2001). Genetic control of the mouse cerebellum: identification of quantitative trait loci modulating size and architecture. J Neurosci 21: 5099–5109.
Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF (2005). Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci 8: 365–371.
American Psychiatric Association 1994. Diagnostic and Statistical Manual of Mental Disorders IV. American Psychiatric Press: Washington, DC.
Anglada-Figueroa D, Quirk GJ (2005). Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci 25: 9680–9685.
Aronsson M, Fuxe K, Dong Y, Agnati LF, Okret S, Gustafsson JA (1988). Localization of glucocorticoid receptor mRNA in the male rat brain by in situ hybridization. Proc Natl Acad Sci USA 85: 9331–9335.
Beaulieu S, Di Paolo T, Barden N (1986). Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology 44: 247–254.
Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK et al (2001). Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry 158: 1248–1251.
Boyce-Rustay JM, Cameron HA, Holmes A (2007). Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol Behav 91: 77–86.
Boyce-Rustay JM, Holmes A (2006). Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology 31: 2405–2414.
Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C et al (1997). Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol Psychiatry 41: 23–32.
Caldarone B, Saavedra C, Tartaglia K, Wehner JM, Dudek BC, Flaherty L (1997). Quantitative trait loci analysis affecting contextual conditioning in mice. Nat Genet 17: 335–337.
Caspi A, Moffitt TE (2006). Gene–environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci 7: 583–590.
Chesler EJ, Wang J, Lu L, Qu Y, Manly KF, Williams RW (2003). Genetic correlates of gene expression in recombinant inbred strains: a relational model system to explore neurobehavioral phenotypes. Neuroinformatics 1: 343–357.
Crawley JN (1981). Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol Biochem Behav 15: 695–699.
Davis M, Whalen PJ (2001). The amygdala: vigilance and emotion. Mol Psychiatry 6: 13–34.
De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G (2001). A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry 50: 305–309.
De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J et al (2002). Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry 52: 1066–1078.
de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG (2006). Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res 40: 550–567.
Decker MW, Curzon P, Brioni JD (1995). Influence of separate and combined septal and amygdala lesions on memory, acoustic startle, anxiety, and locomotor activity in rats. Neurobiol Learn Mem 64: 156–168.
Dunn JD, Whitener J (1986). Plasma corticosterone responses to electrical stimulation of the amygdaloid complex: cytoarchitectural specificity. Neuroendocrinology 42: 211–217.
Fanselow MS, LeDoux JE (1999). Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23: 229–232.
Fanselow MS, Poulos AM (2005). The neuroscience of mammalian associative learning. Annu Rev Psychol 56: 207–234.
Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL (2002). Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry 52: 1089–1101.
Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP et al (2002). Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 5: 1242–1247.
Goosens KA, Maren S (2001). Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem 8: 148–155.
Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S et al (2006). Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA 103: 13208–13213.
Gurvits TV, Metzger LJ, Lasko NB, Cannistraro PA, Tarhan AS, Gilbertson MW et al (2006). Subtle neurologic compromise as a vulnerability factor for combat-related posttraumatic stress disorder: results of a twin study. Arch Gen Psychiatry 63: 571–576.
Handley SL, Mithani S (1984). Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol 327: 1–5.
Hariri AR, Drabant EM, Weinberger DR (2006). Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry 59: 888–897.
Hariri AR, Holmes A (2006). Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci 10: 182–191.
Hefner K, Holmes A (2007). Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res 176: 210–215.
Herman JP, Ostrander MM, Mueller NK, Figueiredo H (2005). Limbic system mechanisms of stress regulation: hypothalamo–pituitary–adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29: 1201–1213.
Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K et al (2007). Processing of temporal unpredictability in human and animal amygdala. J Neurosci 27: 5958–5966.
Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L et al (2003). Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology 28: 1031–1044.
Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM (2004). Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem 81: 67–74.
Izquierdo A, Wellman CL, Holmes A (2006). Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci 26: 5733–5738.
Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A (2006). A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev 30: 1004–1031.
Kendler KS (2001). Twin studies of psychiatric illness: an update. Arch Gen Psychiatry 58: 1005–1014.
Kim JJ, Fanselow MS (1992). Modality-specific retrograde amnesia of fear. Science 256: 675–677.
Kliethermes CL, Crabbe JC (2006). Genetic independence of mouse measures of some aspects of novelty seeking. Proc Natl Acad Sci USA 103: 5018–5023.
Koo JW, Han JS, Kim JJ (2004). Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci 24: 7654–7662.
Lin CH, Hansen S, Wang Z, Storm DR, Tapscott SJ, Olson JM (2005). The dosage of the neuroD2 transcription factor regulates amygdala development and emotional learning. Proc Natl Acad Sci USA 102: 14877–14882.
Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB et al (2004). Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry 56: 356–363.
Lu L, Airey DC, Williams RW (2001). Complex trait analysis of the hippocampus: mapping and biometric analysis of two novel gene loci with specific effects on hippocampal structure in mice. J Neurosci 21: 3503–3514.
Maren S, Quirk GJ (2004). Neuronal signalling of fear memory. Nat Rev Neurosci 5: 844–852.
Meyer-Lindenberg A, Buckholtz JW, Kolachana B, A RH, Pezawas L, Blasi G et al (2006). Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA 103: 6269–6274.
Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S (2005). Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA 102: 9371–9376.
Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M (1997). Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res 760: 94–101.
Moreira CM, Masson S, Carvalho MC, Brandao ML (2007). Exploratory behaviour of rats in the elevated plus-maze is differentially sensitive to inactivation of the basolateral and central amygdaloid nuclei. Brain Res Bull 71: 466–474.
Mozhui K, Hamre KM, Holmes A, Lu L, Williams RW (2007). Genetic and structural analysis of the basolateral amygdala complex in BXD recombinant inbred mice. Behav Genet 37: 223–243.
Nader K, Majidishad P, Amorapanth P, LeDoux JE (2001). Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem 8: 156–163.
Neumann PE, Garretson JD, Skabardonis GP, Mueller GG (1993). Genetic analysis of cerebellar folial pattern in crosses of C57BL/6J and DBA/2J inbred mice. Brain Res 619: 81–88.
Owen EH, Christensen SC, Paylor R, Wehner JM (1997). Identification of quantitative trait loci involved in contextual and auditory-cued fear conditioning in BXD recombinant inbred strains. Behav Neurosci 111: 292–300.
Peirce JL, Chesler EJ, Williams RW, Lu L (2003). Genetic architecture of the mouse hippocampus: identification of gene loci with selective regional effects. Genes Brain Behav 2: 238–252.
Peri T, Ben-Shakhar G, Orr SP, Shalev AY (2000). Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiatry 47: 512–519.
Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS et al (2005). 5-HTTLPR polymorphism impacts human cingulate–amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 8: 828–834.
Phelps EA, LeDoux JE (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48: 175–187.
Porsolt RD, Bertin A, Jalfre M (1978). ‘Behavioural despair’ in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol 51: 291–294.
Quirk GJ, Mueller D (2007). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72.
Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR et al (2006). Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 16: 313–320.
Rauch SL, Shin LM, Phelps EA (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry 60: 376–382.
Redgate ES, Fahringer EE (1973). A comparison of the pituitary adrenal activity elicited by electrical stimulation of preoptic, amygdaloid and hypothalamic sites in the rat brain. Neuroendocrinology 12: 334–343.
Ressler KJ, Mayberg HS (2007). Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10: 1116–1124.
Robbins TW, Everitt BJ (2002). Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem 78: 625–636.
Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, Weinberger NM (2006). Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol Learn Mem 86: 249–255.
Rosen GD, Williams RW (2001). Complex trait analysis of the mouse striatum: independent QTLs modulate volume and neuron number. BMC Neurosci 2: 5.
Seecharan DJ, Kulkarni AL, Lu L, Rosen GD, Williams RW (2003). Genetic control of interconnected neuronal populations in the mouse primary visual system. J Neurosci 23: 11178–11188.
Shimazoe T, Shibata S, Yatsugi S, Ueki S (1988). Involvement of the medial amygdaloid nucleus in the action of imipramine in rats subjected to the forced swimming test. J Pharmacobiodyn 11: 137–139.
Shin LM, Rauch SL, Pitman RK (2006). Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann NY Acad Sci 1071: 67–79.
Swanson LW, Petrovich GD (1998). What is the amygdala? Trends Neurosci 21: 323–331.
Taylor BA (1978). Recombinant inbred strains: use in gene mapping. In: Morse HC (ed). Origins of Inbred Mice. Academic Press: New York. pp 423–438.
Turri MG, Talbot CJ, Radcliffe RA, Wehner JM, Flint J (1999). High-resolution mapping of quantitative trait loci for emotionality in selected strains of mice. Mamm Genome 10: 1098–1101.
Valentinuzzi VS, Kolker DE, Vitaterna MH, Shimomura K, Whiteley A, Low-Zeddies S et al (1998). Automated measurement of mouse freezing behavior and its use for quantitative trait locus analysis of contextual fear conditioning in (BALB/cJ × C57BL/6J)F2 mice. Learn Mem 5: 391–403.
Van de Kar LD, Piechowski RA, Rittenhouse PA, Gray TS (1991). Amygdaloid lesions: differential effect on conditioned stress and immobilization-induced increases in corticosterone and renin secretion. Neuroendocrinology 54: 89–95.
Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22: 6810–6818.
Wahlsten D, Bachmanov A, Finn DA, Crabbe JC (2006). Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci USA 103: 16364–16369.
Wehner JM, Radcliffe RA, Rosmann ST, Christensen SC, Rasmussen DL, Fulker DW et al (1997). Quantitative trait locus analysis of contextual fear conditioning in mice. Nat Genet 17: 331–334.
Wellman CL (2001). Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol 49: 245–253.
Wellman CL, Izquierdo A, Garret JE, Martin KP, Carroll J, Millstein R et al (2007). Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci 27: 684–691.
Wiedholz LM, Owens WA, Horton RE, Feyder M, Karlsson RM, Hefner K et al (2007). Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol Psychiatry.
Wignall EL, Dickson JM, Vaughan P, Farrow TF, Wilkinson ID, Hunter MD et al (2004). Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biol Psychiatry 56: 832–836.
Williams RW (2000). Mapping genes that modulate mouse brain development: a quantitative genetic approach. Results Probl Cell Differ 30: 21–49.
Williams RW, Airey DC, Kulkarni A, Zhou G, Lu L (2001). Genetic dissection of the olfactory bulbs of mice: QTLs on four chromosomes modulate bulb size. Behav Genet 31: 61–77.
Yehuda R (2002). Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am 25: 341–368.
Zorawski M, Killcross S (2002). Posttraining glucocorticoid receptor agonist enhances memory in appetitive and aversive Pavlovian discrete-cue conditioning paradigms. Neurobiol Learn Mem 78: 458–464.
Acknowledgements
We thank Dr. Glenn D. Rosen for access to a new striatal volume data set on the BXD strains available in GeneNetwork (BXD Phenotype GNID 10710). This research was supported by the NIAAA (Z01-AA000411) and NIMH (Z01-MH002784) intramural research programs, and by NIDA, NIMH, and NIAAA HPG grant P20-DA 21131, NCRR BIRN grant U24 RR021760 (BIRN), and NIAAA INIA grants U01AA13499 and U24AA13513.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICT OF INTEREST
The authors have no competing interests.
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Rights and permissions
About this article
Cite this article
Yang, R., Mozhui, K., Karlsson, RM. et al. Variation in Mouse Basolateral Amygdala Volume is Associated With Differences in Stress Reactivity and Fear Learning. Neuropsychopharmacol 33, 2595–2604 (2008). https://doi.org/10.1038/sj.npp.1301665
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301665
Keywords
This article is cited by
-
Stressed rats fail to exhibit avoidance reactions to innately aversive social calls
Neuropsychopharmacology (2022)
-
Evaluation of brain structure and metabolism in currently depressed adults with a history of childhood trauma
Translational Psychiatry (2022)
-
Larger dentate gyrus volume as predisposing resilience factor for the development of trauma-related symptoms
Neuropsychopharmacology (2021)
-
Trauma and posttraumatic stress disorder modulate polygenic predictors of hippocampal and amygdala volume
Translational Psychiatry (2021)
-
The association of PTSD symptom severity with amygdala nuclei volumes in traumatized youths
Translational Psychiatry (2020)