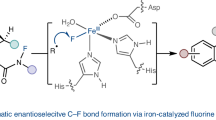
A fluorinase enzyme has been discovered that catalyses carbon–fluorine bond formation.
Abstract
Although fluorine in the form of fluoride minerals is the most abundant halogen in the Earth's crust, only 12 naturally occurring organofluorine compounds have so far been found1, and how these are biosynthesized remains a mystery2. Here we describe an enzymatic reaction that occurs in the bacterium Streptomyces cattleya and which catalyses the conversion of fluoride ion and S-adenosylmethionine (SAM) to 5′-fluoro-5′-deoxyfluoroadenosine (5′-FDA). To our knowledge, this is the first fluorinase enzyme to be identified, a discovery that opens up a new biotechnological opportunity for the preparation of organofluorine compounds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
O'Hagan, D. & Harper, D. B. Nat. Prod. Rep. 11, 123–133 (1994).
O'Hagan, D. & Harper, D. B. in Asymmetric Fluoro-organic Chemistry (ed. Ramachandran, P. V.) 210–225 (Am. Chem. Soc. Symp. Ser. 746, Washington DC, 1999).
Gribble, G. W. Chem. Soc. Rev. 28, 335–346 (1999).
Hall, R. J. New Phytol. 71, 855–871 (1972).
Peters, R. A. & Shorthouse, M. A. Nature 216, 80–81 (1967).
Sanada, M. et al. J. Antibiot. 39, 259–265 (1986).
Moss, S. J. Chem. Commun. 2281–2282 (2000).
Murphy, C. D., Moss, J. S. & O'Hagan, D. Appl. Environ. Microbiol. 67, 4919–4921 (2001).
Gani, D. & Johnson, A. W. J. Chem. Soc. Perkin Trans. 1, 1197–1204 (1982).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
O'Hagan, D., Schaffrath, C., Cobb, S. et al. Biosynthesis of an organofluorine molecule. Nature 416, 279 (2002). https://doi.org/10.1038/416279a
Issue date:
DOI: https://doi.org/10.1038/416279a
This article is cited by
-
Engineering non-haem iron enzymes for enantioselective C(sp3)–F bond formation via radical fluorine transfer
Nature Synthesis (2024)
-
Regioselective, catalytic 1,1-difluorination of enynes
Nature Chemistry (2023)
-
Whole-cell catalysis by surface display of fluorinase on Escherichia coli using N-terminal domain of ice nucleation protein
Microbial Cell Factories (2021)
-
Closing the gap between 19F and 18F chemistry
EJNMMI Radiopharmacy and Chemistry (2021)
-
Enzymatic synthesis of fluorinated compounds
Applied Microbiology and Biotechnology (2021)