Abstract
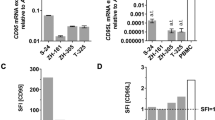
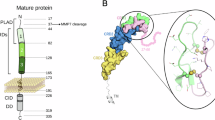
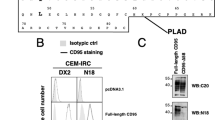
The CD95 (Apo-1/Fas)/CD95 ligand (CD95L) system is best characterized as a trigger of apoptosis. Nevertheless, despite broad expression of CD95L and CD95 in the developing brain, absence of functional CD95 (lpr mice) or CD95L (gld mice) does not alter neuronal numbers. Here, we report that in embryonic hippocampal and cortical neurons in vivo and in vitro CD95L does not induce apoptosis. Triggering of CD95 in cultured immature neurons substantially increases neurite branches by promoting their formation. The branching increase occurs in a caspase-independent and death domain-dependent manner and is paralleled by an increase in the nonphosphorylated form of Tau. Most importantly, lpr and gld mutants exhibit a reduced number of dendritic branches in vivo at the time when synapse formation takes place. These data reveal a novel function for the CD95 system and add to the picture of guidance molecules in the developing brain.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- lpr :
-
lymphoproliferative
- gld :
-
generalized lymphoproliferation
- TUNEL:
-
terminal dUTP nick end labeling
- LZ-CD95:
-
leucine-zipper-CD95L
- DIV:
-
days in vitro
- MTs:
-
microtubules
- Hc:
-
hippocampus
- Cx:
-
cortex
References
Demjen D, Klussmann S, Kleber S, Zuliani C, Stieltjes B, Metzger C, Hirt UA, Walczak H, Falk W, Essig M, Edler L, Krammer PH and Martin-Villalba A (2004) Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat. Med. 10: 389–395
Martin-Villalba A, Herr I, Jeremias I, Hahne M, Brandt R, Vogel J, Schenkel J, Herdegen T and Debatin KM (1999) CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis- inducing ligand mediate ischemia-induced apoptosis in neurons. J. Neurosci. 19: 3809–3817
Martin-Villalba A, Hahne M, Kleber S, Vogel J, Falk W, Schenkel J and Krammer PH (2001) Therapeutic neutralization of CD95L and TNF attentuates brain damage in stroke. Cell Death Differ. 8: 679–686
Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH and Peter ME (1995) Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 14: 5579–5588
Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH and Peter ME (1997) FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16: 2794–2804
Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH and Wallach D (1995) A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 270: 7795–7798
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH and Peter ME (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17: 1675–1687
Desbarats J, Birge RB, Mimouni-Rongy M, Weinstein DE, Palerme JS and Newell MK (2003) Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat. Cell Biol. 5: 118–125
Park C, Sakamaki K, Tachibana O, Yamashima T, Yamashita J and Yonehara S (1998) Expression of fas antigen in the normal mouse brain. Biochem. Biophys. Res. Commun. 252: 623–628
Shin DH, Lee E, Kim HJ, Kim S, Cho SS, Chang KY and Lee WJ (2002) Fas ligand mRNA expression in the mouse central nervous system. J. Neuroimmunol. 123: 50–57
Cheema ZF, Wade SB, Sata M, Walsh K, Sohrabji F and Miranda RC (1999) Fas/Apo [apoptosis]-1 and associated proteins in the differentiating cerebral cortex: induction of caspase-dependent cell death and activation of NF-kappaB. J. Neurosci. 19: 1754–1770
Cheema ZF, Santillano DR, Wade SB, Newman JM and Miranda RC (2004) The extracellular matrix, p53 and estrogen compete to regulate cell-surface Fas/Apo-1 suicide receptor expression in proliferating embryonic cerebral cortical precursors, and reciprocally, Fas-ligand modifies estrogen control of cell-cycle proteins. BMC Neurosci. 5: 11
Ceccatelli S, Tamm C, Sleeper E and Orrenius S (2004) Neural stem cells and cell death. Toxicol. Lett. 149: 59–66
Ricci-Vitiani L, Pedini F, Mollinari C, Condorelli G, Bonci D, Bez A, Colombo A, Parati E, Peschle C and De Maria R (2004) Absence of caspase 8 and high expression of PED protect primitive neural cells from cell death. J. Exp. Med. 200: 1257–1266
Oppenheim RW, Flavell RA, Vinsant S, Prevette D, Kuan CY and Rakic P (2001) Programmed cell death of developing mammalian neurons after genetic deletion of caspases. J. Neurosci. 21: 4752–4760
Cohen PL and Eisenberg RA (1992) The lpr and gld genes in systemic autoimmunity: life and death in the Fas lane. Immunol Today 13: 427–428
Kovac AD, Grammig J, Mahlo J, Steiner B, Roth K, Nitsch R and Bechmann I (2002) Comparison of neuronal density and subfield sizes in the hippocampus of CD95L-deficient (gld), CD95-deficient (lpr) and nondeficient mice. Eur. J. Neurosci. 16: 159–163
Adachi M, Watanabe-Fukunaga R and Nagata S (1993) Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc. Natl. Acad. Sci. USA 90: 1756–1760
Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA and Nagata S (1992) Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356: 314–317
Cleveland DW, Hwo SY and Kirschner MW (1977) Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J. Mol. Biol. 116: 207–225
Drubin DG and Kirschner MW (1986) Tau protein function in living cells. J. Cell Biol. 103: 2739–2746
Schneider A, Biernat J, von Bergen M, Mandelkow E and Mandelkow EM (1999) Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 38: 3549–3558
Schwab C, DeMaggio AJ, Ghoshal N, Binder LI, Kuret J and McGeer PL (2000) Casein kinase 1 delta is associated with pathological accumulation of tau in several neurodegenerative diseases. Neurobiol. Aging 21: 503–510
Smith HR and Steinberg AD (1983) Autoimmunity – a perspective. Ann. Rev. Immunol. 1: 175–210
Sakic B, Szechtman H, Denburg JA, Gorny G, Kolb B and Whishaw IQ (1998) Progressive atrophy of pyramidal neuron dendrites in autoimmune MRL-lpr mice. J. Neuroimmunol. 87: 162–170
Raoul C, Henderson CE and Pettmann B (1999) Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J. Cell Biol. 147: 1049–1062
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B and Tschopp J (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1: 489–495
Wagner U, Utton M, Gallo JM and Miller CC (1996) Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. J. Cell Sci. 109: 1537–1543
Richter JD (2001) Think globally, translate locally: what mitotic spindles and neuronal synapses have in common. Proc. Natl. Acad. Sci. USA 98: 7069–7071
Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T and Nagata S (1994) Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76: 969–976
Sneller MC, Straus SE, Jaffe ES, Jaffe JS, Fleisher TA, Stetler-Stevenson M and Strober W (1992) A novel lymphoproliferative/autoimmune syndrome resembling murine lpr/gld disease. J. Clin. Invest. 90: 334–341
Hess DC, Taormina M, Thompson J, Sethi KD, Diamond B, Rao R, Chamberlain CR and Feldman DS (1993) Cognitive and neurologic deficits in the MRL/lpr mouse: a clinicopathologic study. J. Rheumatol. 20: 610–617
Feinglass EJ, Arnett FC, Dorsch CA, Zizic TM and Stevens MB (1976) Neuropsychiatric manifestations of systemic lupus erythematosus: diagnosis, clinical spectrum, and relationship to other features of the disease. Medicine (Baltimore) 55: 323–339
Johnson RT and Richardson EP (1968) The neurological manifestations of systemic lupus erythematosus. Medicine (Baltimore) 47: 337–369
Denburg SD and Denburg JA (2003) Cognitive dysfunction and antiphospholipid antibodies in systemic lupus erythematosus. Lupus 12: 883–890
Denburg JA, Carbotte RM and Denburg SD (1987) Neuronal antibodies and cognitive function in systemic lupus erythematosus. Neurology 37: 464–467
Denburg SD, Carbotte RM, Ginsberg JS and Denburg JA (1997) The relationship of antiphospholipid antibodies to cognitive function in patien1ts with systemic lupus erythematosus. J. Int. Neuropsychol. Soc. 3: 377–386
Denburg JA, Carbotte RM and Denburg SD (1987) Neuronal antibodies and cognitive function in systemic lupus erythematosus. Neurology 37: 464–467
Banker G and Goslin K (1988) Developments in neuronal cell culture. Nature 336: 185–186
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC and Lynch DH (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis- inducing ligand in vivo [see comments]. Nat. Med. 5: 157–163
Naldini L, Blomer U, Gage FH, Trono D and Verma IM (1996) Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. USA 93: 11382–11388
Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L and Trono D (1998) Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 72: 9873–9880
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F and Riccardi C (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139: 271–279
Ernst T, Hergenhahn M, Kenzelmann M, Cohen CD, Bonrouhi M, Weninger A, Klaren R, Grone EF, Wiesel M, Gudemann C, Kuster J, Schott W, Staehler G, Kretzler M, Hollstein M and Grone HJ (2002) Decrease and gain of gene expression are equally discriminatory markers for prostate carcinoma: a gene expression analysis on total and microdissected prostate tissue. Am. J. Pathol. 160: 2169–2180
Kenzelmann M, Klaren R, Hergenhahn M, Bonrouhi M, Grone HJ, Schmid W and Schutz G (2004) High-accuracy amplification of nanogram total RNA amounts for gene profiling. Genomics 83: 550–558
Acknowledgements
We thank Inna Lavrik and Dagmar Riess for LZ-CD95L preparation, and Alexandra Beisel and Claudia Schmidt for technical assistance. We thank Jörg Tschopp for providing us with the lpr and gld mutant mice. This work was supported by the Christopher Reeve Paralysis Foundation (Grant KAC2-0101-2), an SFB 405 Grant (DFG, Germany), and a SAF2001-3290 Grant (Spanish Ministry of Science and Technology).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by P Nicotera
This paper is dedicated to M. Hergenhahn (✠).
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Zuliani, C., Kleber, S., Klussmann, S. et al. Control of neuronal branching by the death receptor CD95 (Fas/Apo-1). Cell Death Differ 13, 31–40 (2006). https://doi.org/10.1038/sj.cdd.4401720
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401720
Keywords
This article is cited by
-
How CD40L reverse signaling regulates axon and dendrite growth
Cellular and Molecular Life Sciences (2021)
-
sCD95L in serum after spinal cord injury
Spinal Cord (2016)
-
Mice lacking functional CD95-ligand display reduced proliferation of the intestinal epithelium without gross homeostatic alterations
Medical Molecular Morphology (2016)
-
The role of CD95 and CD95 ligand in cancer
Cell Death & Differentiation (2015)
-
Amyloid-β reduces the expression of neuronal FAIM-L, thereby shifting the inflammatory response mediated by TNFα from neuronal protection to death
Cell Death & Disease (2015)