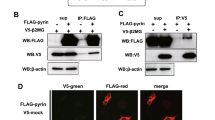
Abstract
Mutations in cryopyrin and pyrin proteins are responsible for several autoinflammatory disorders in humans, suggesting that these proteins play important roles in regulating inflammation. Using a HEK293 cell-based reconstitution system that stably expresses ASC and procaspase-1 we demonstrated that neither cryopyrin nor pyrin or their corresponding disease-associated mutants could significantly activate NF-κB in this system. However, both cryopyrin and two disease-associated cryopyrin mutants induced ASC oligomerization and ASC-dependent caspase-1 activation, with the disease-associated mutants being more potent than the wild-type (WT) cryopyrin, because of increased self-oligomerization. Contrary to the proposed anti-inflammatory activity of WT pyrin, our results demonstrated that pyrin, like cryopyrin, can also assemble an inflammasome complex with ASC and procaspase-1 leading to ASC oligomerization, caspase-1 activation and interleukin-1β processing. Thus, we propose that pyrin could function as a proinflammatory molecule.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CARD:
-
caspase-associated recruitment domain
- PYD:
-
pyrin domain
- DD:
-
death domain
- DED:
-
death effector domain
- NOD:
-
nucleotide-binding oligomerization domain
- LRR:
-
leucine-rich repeat
- NLR:
-
NACHT-LRR
- IL:
-
interleukin
- LPS:
-
lipopolysaccharide
- TNF:
-
tumor necrosis factor
- ASC:
-
apoptosis-associated speck-like protein containing a CARD
- PFS:
-
periodic fever syndromes
- FMF:
-
familial Mediterranean fever
- FCAS:
-
familial cold autoinflammatory syndrome
- FCU:
-
familial cold urticaria
- MWS:
-
Muckle-Wells syndrome
- NOMID:
-
neonatal-onset multisystem inflammatory disease
- CINCA:
-
chronic infantile neurological cutaneous and articular syndrome
- PSTPIP1:
-
proline serine threonine phosphatase-interacting protein 1
- PAPA:
-
pyogenic arthritis, pyoderma gangrenosum, and acne syndrome
- IKK:
-
IκB kinase
- PAMP:
-
pathogen-associated molecular patterns
- PGN:
-
peptidoglycans
- MDP:
-
muramyl dipeptide
- WT:
-
wildtype
- RT:
-
reverse transcription
- PCR:
-
polymerase chain reaction
- NACHT:
-
domain present in NAIP, CIITA, HET-E and TP1
References
Gumucio DL, Diaz A, Schaner P, Richards N, Babcock C, Schaller M and Cesena T (2002) Fire and ICE: the role of pyrin domain-containing proteins in inflammation and apoptosis. Clin. Exp. Rheumatol. 20: S45–S53
Hull KM, Shoham N, Chae JJ, Aksentijevich I and Kastner DL (2003) The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr. Opin. Rheumatol. 15: 61–69
McDermott MF and Aksentijevich I (2002) The autoinflammatory syndromes. Curr. Opin. Allergy Clin. Immunol. 2: 511–516
Stehlik C and Reed JC (2004) The PYRIN connection: novel players in innate immunity and inflammation. J. Exp. Med. 200: 551–558
Tschopp J, Martinon F and Burns K (2003) NALPs: a novel protein family involved in inflammation. Nat. Rev. Mol. Cell Biol. 4: 95–104
Consortium TFF (1997) A candidate gene for familial Mediterranean fever. The French FMF Consortium. Nat. Genet. 17: 25–31
Consortium TIF (1997) Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell 90: 797–807
Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, Teillac-Hamel D, Fischer A and de Saint Basile G (2002) Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 71: 198–203
Hoffman HM, Mueller JL, Broide DH, Wanderer AA and Kolodner RD (2001) Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nat. Genet. 29: 301–305
Centola M, Wood G, Frucht DM, Galon J, Aringer M, Farrell C, Kingma DW, Horwitz ME, Mansfield E, Holland SM, O'Shea JJ, Rosenberg HF, Malech HL and Kastner DL (2000) The gene for familial Mediterranean fever, MEFV, is expressed in early leukocyte development and is regulated in response to inflammatory mediators. Blood 95: 3223–3231
Chae JJ, Komarow HD, Cheng J, Wood G, Raben N, Liu PP and Kastner DL (2003) Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol. Cell 11: 591–604
Bertin J and DiStefano PS (2000) The PYRIN domain: a novel motif found in apoptosis and inflammation proteins. Cell Death Differ. 7: 1273–1274
Fairbrother WJ, Gordon NC, Humke EW, O'Rourke KM, Starovasnik MA, Yin JP and Dixit VM (2001) The PYRIN domain: a member of the death domain-fold superfamily. Protein Sci. 10: 1911–1918
Martinon F, Hofmann K and Tschopp J (2001) The pyrin domain: a possible member of the death domain-fold family implicated in apoptosis and inflammation. Curr. Biol. 11: R118–R120
Pawlowski K, Pio F, Chu Z, Reed JC and Godzik A (2001) PAAD – a new protein domain associated with apoptosis, cancer and autoimmune diseases. Trends Biochem. Sci. 26: 85–87
Schaner P, Richards N, Wadhwa A, Aksentijevich I, Kastner D, Tucker P and Gumucio D (2001) Episodic evolution of pyrin in primates: human mutations recapitulate ancestral amino acid states. Nat. Genet. 27: 318–321
Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, Hidaka E, Katsuyama T, Higuchi T and Sagara J (1999) ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J. Biol. Chem. 274: 33835–33838
Richards N, Schaner P, Diaz A, Stuckey J, Shelden E, Wadhwa A and Gumucio DL (2001) Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J. Biol. Chem. 276: 39320–39329
Martinon F, Burns K and Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10: 417–426
Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z and Alnemri ES (2002) The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem. 277: 21119–21122
Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, Geddes BJ, Briskin M, DiStefano PS and Bertin J (2002) PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J. Biol. Chem. 277: 29874–29880
Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S and Dixit VM (2004) Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430: 213–218
Shoham NG, Centola M, Mansfield E, Hull KM, Wood G, Wise CA and Kastner DL (2003) Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl. Acad. Sci. USA 100: 13501–13506
Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S and Lovett M (2002) Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum. Mol. Genet. 11: 961–969
McDermott MF (2004) A common pathway in periodic fever syndromes. Trends Immunol. 25: 457–460
Ting JP and Davis BK (2005) Caterpiller: a novel gene family important in immunity, cell death, and diseases. Annu. Rev. Immunol. 23: 387–414
McDermott MF (2002) Genetic clues to understanding periodic fevers, and possible therapies. Trends Mol. Med. 8: 550–554
Anderson JP, Mueller JL, Rosengren S, Boyle DL, Schaner P, Cannon SB, Goodyear CS and Hoffman HM (2004) Structural, expression, and evolutionary analysis of mouse CIAS1. Gene 338: 25–34
Manji GA, Wang L, Geddes BJ, Brown M, Merriam S, Al-Garawi A, Mak S, Lora JM, Briskin M, Jurman M, Cao J, DiStefano PS and Bertin J (2002) PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-kappa B. J. Biol. Chem. 277: 11570–11575
Dowds TA, Masumoto J, Chen FF, Ogura Y, Inohara N and Nunez G (2003) Regulation of cryopyrin/Pypaf1 signaling by pyrin, the familial Mediterranean fever gene product. Biochem. Biophys. Res. Commun. 302: 575–580
Dowds TA, Masumoto J, Zhu L, Inohara N and Nunez G (2004) Cryopyrin-induced interleukin 1beta secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J. Biol. Chem. 279: 21924–21928
O'Connor Jr W, Harton JA, Zhu X, Linhoff MW and Ting JP (2003) Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1.1 is an inducible inflammatory mediator with NF-kappa B suppressive properties. J. Immunol. 171: 6329–6333
Stehlik C, Fiorentino L, Dorfleutner A, Bruey JM, Ariza EM, Sagara J and Reed JC (2002) The PAAD/PYRIN-family protein ASC is a dual regulator of a conserved step in nuclear factor kappaB activation pathways. J. Exp. Med. 196: 1605–1615
Karin M and Delhase M (2000) The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin. Immunol. 12: 85–98
Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN and Tschopp J (2004) NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity 20: 319–325
Martinon F, Agostini L, Meylan E and Tschopp J (2004) Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr. Biol. 14: 1929–1934
Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T and Alnemri ES (2001) Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 276: 28309–28313
Neven B, Callebaut I, Prieur AM, Feldmann J, Bodemer C, Lepore L, Derfalvi B, Benjaponpitak S, Vesely R, Sauvain MJ, Oertle S, Allen R, Morgan G, Borkhardt A, Hill C, Gardner-Medwin J, Fischer A and de Saint Basile G (2004) Molecular basis of the spectral expression of CIAS1 mutations associated with phagocytic cell-mediated autoinflammatory disorders CINCA/NOMID, MWS, and FCU. Blood 103: 2809–2815
Yamamoto M, Yaginuma K, Tsutsui H, Sagara J, Guan X, Seki E, Yasuda K, Akira S, Nakanishi K, Noda T and Taniguchi S (2004) ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells 9: 1055–1067
Henry J, Mather IH, McDermott MF and Pontarotti P (1998) B30.2-like domain proteins: update and new insights into a rapidly expanding family of proteins. Mol. Biol. Evol. 15: 1696–1705
Schultz J, Milpetz F, Bork P and Ponting CP (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95: 5857–5864
Aksentijevich I, Torosyan Y, Samuels J, Centola M, Pras E, Chae JJ, Oddoux C, Wood G, Azzaro MP, Palumbo G, Giustolisi R, Pras M, Ostrer H and Kastner DL (1999) Mutation and haplotype studies of familial Mediterranean fever reveal new ancestral relationships and evidence for a high carrier frequency with reduced penetrance in the Ashkenazi Jewish population. Am. J. Hum. Genet. 64: 949–962
Stoffman N, Magal N, Shohat T, Lotan R, Koman S, Oron A, Danon Y, Halpern GJ, Lifshitz Y and Shohat M (2000) Higher than expected carrier rates for familial Mediterranean fever in various Jewish ethnic groups. Eur. J. Hum. Genet. 8: 307–310
Tunca M, Kirkali G, Soyturk M, Akar S, Pepys MB and Hawkins PN (1999) Acute phase response and evolution of familial Mediterranean fever. Lancet 353: 1415
Mansfield E, Chae JJ, Komarow HD, Brotz TM, Frucht DM, Aksentijevich I and Kastner DL (2001) The familial Mediterranean fever protein, pyrin, associates with microtubules and colocalizes with actin filaments. Blood 98: 851–859
Acknowledgements
We thank Dr. Gabriel Nunez for the WT and mutant cryopyrin expression plasmids and Dr. Daniel Kastner for the WT and mutant pyrin expression plasmids. I Ibrahimi is on sabbatical leave from the University of Jordan. He is partially supported by the distinguished scholar award from the Arab fund for social and economic development. This work was supported by NIH grants AG14357 and AG13487 (to ESA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by DR Green
Supplementary information accompanies the paper on the Cell Death and Differentiation web site(http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Yu, JW., Wu, J., Zhang, Z. et al. Cryopyrin and pyrin activate caspase-1, but not NF-κB, via ASC oligomerization. Cell Death Differ 13, 236–249 (2006). https://doi.org/10.1038/sj.cdd.4401734
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401734
Keywords
This article is cited by
-
Recent Insights in Pyrin Inflammasome Activation: Identifying Potential Novel Therapeutic Approaches in Pyrin-Associated Autoinflammatory Syndromes
Journal of Clinical Immunology (2024)
-
Dynamic disequilibrium-based pathogenicity model in mutated pyrin’s B30.2 domain—Casp1/p20 complex
Journal of Genetic Engineering and Biotechnology (2022)
-
Interleukin-1 Antagonists for the Treatment of Recurrent Pericarditis
BioDrugs (2022)
-
The PRY/SPRY domain of pyrin/TRIM20 interacts with β2-microglobulin to promote inflammasome formation
Scientific Reports (2021)
-
Articular manifestations in Egyptian children with familial Mediterranean fever
Egyptian Rheumatology and Rehabilitation (2020)