Abstract
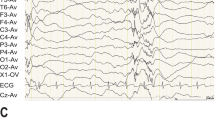
Childhood absence epilepsy (CAE) is an idiopathic generalised epilepsy characterised by absence seizures manifested by transitory loss of awareness with 2.5–4 Hz spike–wave complexes on ictal EEG. A genetic component to aetiology is established but the mechanism of inheritance and the genes involved are not fully defined. Available evidence suggests that genes encoding brain expressed voltage-gated calcium channels, including CACNG3 on chromosome 16p12–p13.1, may represent susceptibility loci for CAE. The aim of this work was to further evaluate CACNG3 as a susceptibility locus by linkage and association analysis. Assuming locus heterogeneity, a significant HLOD score (HLOD=3.54, α=0.62) was obtained for markers encompassing CACNG3 in 65 nuclear families with a proband with CAE. The maximum non-parametric linkage score was 2.87 (P<0.002). Re-sequencing of the coding exons in 59 patients did not identify any putative causal variants. A linkage disequilibrium (LD) map of CACNG3 was constructed using 23 single nucleotide polymorphisms (SNPs). Transmission disequilibrium was sought using individual SNPs and SNP-based haplotypes with the pedigree disequilibrium test in 217 CAE trios and the 65 nuclear pedigrees. Evidence for transmission disequilibrium (P≤0.01) was found for SNPs within a ∼35 kb region of high LD encompassing the 5’UTR, exon 1 and part of intron 1 of CACNG3. Re-sequencing of this interval was undertaken in 24 affected individuals. Seventy-two variants were identified: 45 upstream; two 5’UTR; and 25 intronic SNPs. No coding sequence variants were identified, although four variants are predicted to affect exonic splicing. This evidence supports CACNG3 as a susceptibility locus in a subset of CAE patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Engel Jr J : A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001; 42: 796–803.
Berkovic SF, Andermann F, Andermann E, Gloor P : Concepts of absence epilepsies: discrete syndromes or biological continuum? Neurology 1987; 37: 993–1000.
Marini C, Scheffer IE, Crossland KM et al: Genetic architecture of idiopathic generalized epilepsy: clinical genetic analysis of 55 multiplex families. Epilepsia 2004; 45: 467–478.
Berkovic SF, Howell RA, Hay DA, Hopper JL : Epilepsies in twins: genetics of the major epilepsy syndromes. Ann Neurol 1998; 43: 435–445.
Kjeldsen MJ, Corey LA, Christensen K, Friis ML : Epileptic seizures and syndromes in twins: the importance of genetic factors. Epilepsy Res 2003; 55: 137–146.
Fletcher CF, Lutz CM, O'Sullivan TN et al: Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell 1996; 87: 607–617.
Burgess DL, Jones JM, Meisler MH, Noebels JL : Mutation of the Ca2+ channel beta subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell 1997; 88: 385–392.
Barclay J, Balaguero N, Mione M et al: Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci 2001; 21: 6095–6104.
Letts VA, Felix R, Biddlecome GH et al: The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet 1998; 19: 340–347.
Tomita S, Chen L, Kawasaki Y et al: Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol 2003; 161: 805–816.
Chen L, Chetkovich DM, Petralia RS et al: Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 2000; 408: 936–943.
Sander T, Schulz H, Saar K et al: Genome search for susceptibility loci of common idiopathic generalised epilepsies. Hum Mol Genet 2000; 9: 1465–1472.
Fong GC, Shah PU, Gee MN et al: Childhood absence epilepsy with tonic-clonic seizures and electroencephalogram 3-4-Hz spike and multispike-slow wave complexes: linkage to chromosome 8q24. Am J Hum Genet 1998; 63: 1117–1129.
Haug K, Warnstedt M, Alekov AK et al: Mutations in CLCN2 encoding a voltage-gated chloride channel are associated with idiopathic generalized epilepsies. Nat Genet 2003; 33: 527–532.
Sugimoto Y, Morita R, Amano K et al: Childhood absence epilepsy in 8q24: refinement of candidate region and construction of physical map. Genomics 2000; 68: 264–272.
Wallace RH, Marini C, Petrou S et al: Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet 2001; 28: 49–52.
Chioza B, Wilkie H, Nashef L et al: Association between the alpha(1a) calcium channel gene CACNA1A and idiopathic generalized epilepsy. Neurology 2001; 56: 1245–1246.
Chen Y, Lu J, Pan H et al: Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann Neurol 2003; 54: 239–243.
Heron SE, Phillips HA, Mulley JC et al: Genetic variation of CACNA1H in idiopathic generalized epilepsy. Ann Neurol 2004; 55: 595–596.
Chioza B, Everett K, Aschauer H et al: Evaluation of CACNA1H in European patients with childhood absence epilepsy. Epilepsy Res 2006; 69: 177–181.
Robinson R, Taske N, Sander T et al: Linkage analysis between childhood absence epilepsy and genes encoding GABAA and GABAB receptors, voltage-dependent calcium channels, and the ECA1 region on chromosome 8q. Epilepsy Res 2002; 48: 169–179.
Jeganathan D, Chodhari R, Meeks M et al: Loci for primary ciliary dyskinesia map to chromosome 16p12.1–12.2 and 15q13.1–15.1 in Faroe Islands and Israeli Druze genetic isolates. J Med Genet 2004; 41: 233–240.
O’Connell JR, Weeks DE : PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 1998; 63: 259–266.
Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES : Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 1996; 58: 1347–1363.
Risch N : Linkage strategies for genetically complex traits. I. Multilocus models. Am J Hum Genet 1990; 46: 222–228.
Barrett JC, Fry B, Maller J, Daly MJ : Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–265.
Martin ER, Monks SA, Warren LL, Kaplan NL : A test for linkage and association in general pedigrees: the pedigree disequilibrium test. Am J Hum Genet 2000; 67: 146–154.
Martin ER, Bass MP, Kaplan NL : Correcting for a potential bias in the pedigree disequilibrium test. Am J Hum Genet 2001; 68: 1065–1067.
Pertea M, Lin X, Salzberg SL : GeneSplicer: a new computational method for splice site prediction. Nucleic Acids Res 2001; 29: 1185–1190.
Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR : ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res 2003; 31: 3568–3571.
de Bakker PI, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D : Efficiency and power in genetic association studies. Nat Genet 2005; 37: 1217–1223.
Soranzo N, Cavalleri GL, Weale ME et al: Identifying candidate causal variants responsible for altered activity of the ABCB1 multidrug resistance gene. Genome Res 2004; 14: 1333–1344.
Nyholt DR : A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 2004; 74: 765–769.
Salyakina D, Seaman SR, Browning BL, Dudbridge F, Muller-Myhsok B : Evaluation of Nyholt's procedure for multiple testing correction. Hum Hered 2005; 60: 19–25.
De Gobbi M, Viprakasit V, Hughes JR et al: A regulatory SNP causes a human genetic disease by creating a new transcriptional promoter. Science 2006; 312: 1215–1217.
Lawrence R, Evans DM, Morris AP et al: Genetically indistinguishable SNPs and their influence on inferring the location of disease-associated variants. Genome Res 2005; 15: 1503–1510.
Acknowledgements
This work was supported by the MRC (UK), Wellcome Trust, Action Medical Research and Epilepsy Research Foundation. We are very grateful to the families for participating in this study and to all our 142 collaborating clinicians, including Dr Lina Nashef. We thank Généthon for their assistance in collecting the French samples and Richard Sharp for his technical help. Austrian financial support came from the Austrian Research Foundation (awarded to Harald Aschauer, MD), Grant number P10460-MED. Thomas Sander, MD, was awarded a grant by the Deutsche Forschungsgemeinschaft (Sa434/3-1), the German National Genome Research Network (01GS0479). Dutch financial support came from the Netherlands Organisation for Health, Research and Development (ZonMW, 940-33-030) and the Dutch National Epilepsy Fund – ‘The power of the small’ (NEF – ‘De macht van het kleine’). Danish support (Mogens Friis, MD, and Marianne Kjeldsen, MD) came from the NINDS grant (NS-31564).
Author information
Authors and Affiliations
Corresponding author
Additional information
Web Resources
The URLs for data presented herein are as follows:
Advanced Biotechnology Centre, Imperial College, http://bm-abc01.cx.med.ic.ac.uk/
KBioscience, http://www.kbioscience.co.uk
NCBI Nucleotide Database, http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=nuccore
Polymorphic DNA Technologies, http://www.polymorphicdna.com
Softberry NSITE Portal, http://www.softberry.com
GeneSplicer, http://www.tigr.org/tdb/GeneSplicer/gene_spl.html
ESEFinder, http://rulai.cshl.edu/tools/ESE/
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary information
Rights and permissions
About this article
Cite this article
Everett, K., Chioza, B., Aicardi, J. et al. Linkage and association analysis of CACNG3 in childhood absence epilepsy. Eur J Hum Genet 15, 463–472 (2007). https://doi.org/10.1038/sj.ejhg.5201783
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.ejhg.5201783
Keywords
This article is cited by
-
Integrated profiling identifies CACNG3 as a prognostic biomarker for patients with glioma
BMC Cancer (2023)
-
Ameliorative effect of curcumin on altered expression of CACNA1A and GABRD in the pathogenesis of FeCl3-induced epilepsy
Molecular Biology Reports (2020)
-
An Association Analysis of Reelin Gene (RELN) Polymorphisms with Childhood Epilepsy in Eastern Indian Population from West Bengal
Cellular and Molecular Neurobiology (2011)
-
Genetic basis in epilepsies caused by malformations of cortical development and in those with structurally normal brain
Human Genetics (2009)