Abstract
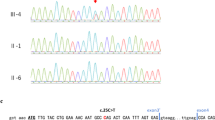
Increases in the number of allelic malformation syndromes have led to their classification according to their pathogenesis rather than their clinical specific phenotype. TP63 (also known as TP73L) mutations have been identified in several such syndromes characterized by autosomal dominant transmission and various combinations of ectodermal dysplasia, limb malformations and orofacial clefting. TP63 has not yet been implicated in early aging phenotype in humans, even though p63 activates a program of cellular senescence and p63-compromised mice display features of accelerated aging. We report on a family with four affected adult females presenting with Rapp–Hodgkin syndrome (RHS), an autosomal dominant clinical entity that associates anhidrotic ectodermal dysplasia with cleft lip and palate. Features between RHS and EEC syndrome (ectrodactyly, ectodermal dysplasia and cleft lip/palate) have led to the recent identification of mutations in the TP63 gene, located on 3q27, in this condition. Our patients present typical clinical features of RHS, but also ophthalmic anomalies such as corneal dystrophy and premature menopause (around 30 years). The latter findings have never been reported in this condition, and could be secondary to a new TP63 deletion that has been identified in this family.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Rapp RS, Hodgkin WE : Anhidrotic ectodermal dysplasia: autosomal dominant inheritance with palate and lip anomalies. J Med Genet 1968; 5: 269–272.
Moerman P, Fryns JP : Ectodermal dysplasia, Rapp–Hodgkin type in a mother and severe ectrodactyly-ectodermal dysplasia-clefting syndrome (EEC) in her child. Am J Med Genet 1996; 63: 479–481.
Cambiaghi S, Tadini G, Barbareschi M, Menni S, Caputo R : Rapp–Hodgkin syndrome and AEC syndrome: are they the same entity? Br J Dermatol 1994; 130: 97–101.
Celli J, Duijf P, Hamel BC et al: Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell 1999; 99: 143–153.
Brunner HG, Hamel BC, Van Bokhoven H : The p63 gene in EEC and other syndromes. J Med Genet 2002; 39: 377–381.
McGrath JA, Duijf PH, Doetsch V et al: Hay–Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63. Hum Mol Genet 2001; 10: 221–229.
van Bokhoven H, Hamel BC, Bamshad M et al: p63 Gene mutations in eec syndrome, limb-mammary syndrome, and isolated split hand-split foot malformation suggest a genotype-phenotype correlation. Am J Hum Genet 2001; 69: 481–492.
Amiel J, Bougeard G, Francannet C et al: TP63 gene mutation in ADULT syndrome. Eur J Hum Genet 2001; 9: 642–645.
Ianakiev P, Kilpatrick MW, Toudjarska I, Basel D, Beighton P, Tsipouras P : Split-hand/split-foot malformation is caused by mutations in the p63 gene on 3q27. Am J Hum Genet 2000; 67: 59–66.
Bougeard G, Hadj-Rabia S, Faivre L, Sarafan-Vasseur N, Frebourg T : The Rapp–Hodgkin syndrome results from mutations of the TP63 gene. Eur J Hum Genet 2003; 11: 700–704.
Leoyklang P, Siriwan P, Shotelersuk V : A mutation of the p63 gene in non-syndromic cleft lip. J Med Genet 2006; 43: e28.
Yang A, Kaghad M, Wang Y et al: p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 1998; 2: 305–316.
Rohmann E, Brunner HG, Kayserili H et al: Mutations in different components of FGF signaling in LADD syndrome. Nat Genet 2006; 38: 414–417.
Keyes WM, Vogel H, Koster MI et al: p63 heterozygous mutant mice are not prone to spontaneous or chemically induced tumors. Proc Natl Acad Sci USA 2006; 103: 8435–8440.
Koster MI, Roop DR : Transgenic mouse models provide new insights into the role of p63 in epidermal development. Cell Cycle 2004; 3: 411–413.
Serber Z, Lai HC, Yang A et al: A C-terminal inhibitory domain controls the activity of p63 by an intramolecular mechanism. Mol Cell Biol 2002; 22: 8601–8611.
Fomenkov A, Huang YP, Topaloglu O et al: P63 alpha mutations lead to aberrant splicing of keratinocyte growth factor receptor in the Hay–Wells syndrome. J Biol Chem 2003; 278: 23906–23914.
van Bokhoven H, Brunner HG : Splitting p63. Am J Hum Genet 2002; 71: 1–13.
Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A : p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999; 398: 708–713.
Pellegrini G, Dellambra E, Golisano O et al: p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA 2001; 98: 3156–3161.
Kurita T, Cunha GR : Roles of p63 in differentiation of Mullerian duct epithelial cells. Ann N Y Acad Sci 2001; 948: 9–12.
Kurita T, Cunha GR, Robboy SJ, Mills AA, Medina RT : Differential expression of p63 isoforms in female reproductive organs. Mech Dev 2005; 122: 1043–1055.
Keyes WM, Wu Y, Vogel H, Guo X, Lowe SW, Mills AA : p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes Dev 2005; 19: 1986–1999.
Suh EK, Yang A, Kettenbach A et al: p63 protects the female germ line during meiotic arrest. Nature 2006; 444: 624–628.
Munier FL, Korvatska E, Djemai A et al: Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nat Genet 1997; 15: 247–251.
Goswami D, Conway GS : Premature ovarian failure. Hum Reprod Update 2005; 11: 391–410.
Di Pasquale E, Beck-Peccoz P, Persani L : Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet 2004; 75: 106–111.
Di Pasquale E, Rossetti R, Marozzi A et al: Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab 2006; 91: 1976–1979.
Bakkers J, Hild M, Kramer C, Furutani-Seiki M, Hammerschmidt M : Zebrafish DeltaNp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Dev Cell 2002; 2: 617–627.
Dianzani I, Garelli E, Gustavsson P et al: Rapp–Hodgkin and AEC syndromes due to a new frameshift mutation in the TP63 gene. J Med Genet 2003; 40: e133.
Bertola DR, Kim CA, Albano LM, Scheffer H, Meijer R, van Bokhoven H : Molecular evidence that AEC syndrome and Rapp–Hodgkin syndrome are variable expression of a single genetic disorder. Clin Genet 2004; 66: 79–80.
Chan I, McGrath JA, Kivirikko S : Rapp–Hodgkin syndrome and the tail of p63. Clin Exp Dermatol 2005; 30: 183–186.
Yang A, Schweitzer R, Sun D et al: p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999; 398: 714–718.
Yang A, McKeon F : P63 and P73: P53 mimics, menaces and more. Nat Rev Mol Cell Biol 2000; 1: 199–207.
Acknowledgements
We are very grateful for the advices that Professor Alea Mills gave us throughout the elaboration of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary information
Rights and permissions
About this article
Cite this article
Holder-Espinasse, M., Martin-Coignard, D., Escande, F. et al. A new mutation in TP63 is associated with age-related pathology. Eur J Hum Genet 15, 1115–1120 (2007). https://doi.org/10.1038/sj.ejhg.5201888
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.ejhg.5201888
Keywords
This article is cited by
-
Heterozygous TP63 pathogenic variants in isolated primary ovarian insufficiency
Journal of Assisted Reproduction and Genetics (2023)
-
Structural diversity of p63 and p73 isoforms
Cell Death & Differentiation (2022)
-
The p63 C-terminus is essential for murine oocyte integrity
Nature Communications (2021)